Chitosan and its antimicrobial potential--a critical literature survey - PubMed (original) (raw)
Review
Chitosan and its antimicrobial potential--a critical literature survey
Dina Raafat et al. Microb Biotechnol. 2009 Mar.
Abstract
Chitosan, an aminopolysaccharide biopolymer, has a unique chemical structure as a linear polycation with a high charge density, reactive hydroxyl and amino groups as well as extensive hydrogen bonding. It displays excellent biocompatibility, physical stability and processability. The term 'chitosan' describes a heterogeneous group of polymers combining a group of physicochemical and biological characteristics, which allow for a wide scope of applications that are both fascinating and as yet uncharted. The increased awareness of the potentials and industrial value of this biopolymer lead to its utilization in many applications of technical interest, and increasingly in the biomedical arena. Although not primarily used as an antimicrobial agent, its utility as an ingredient in both food and pharmaceutical formulations lately gained more interest, when a scientific understanding of at least some of the pharmacological activities of this versatile carbohydrate began to evolve. However, understanding the various factors that affect its antimicrobial activity has become a key issue for a better usage and a more efficient optimization of chitosan formulations. Moreover, the use of chitosan in antimicrobial systems should be based on sufficient knowledge of the complex mechanisms of its antimicrobial mode of action, which in turn would help to arrive at an appreciation of its entire antimicrobial potential.
© 2009 The Authors. Journal compilation © 2009 Society for Applied Microbiology and Blackwell Publishing Ltd.
Figures
Figure 1
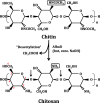
Chemical structure of chitosan, its production from chitin and the specificity of chitosanases. Chitosan is a (1→4)‐linked 2‐amino‐2‐deoxy‐_β_‐
d
‐glucan, prepared from chitin through alkaline hydrolysis of the _N_‐acetyl groups. Upon further hydrolysis, for example, with the help of chitosanases (indicated by black arrows), low‐MW oligosaccharides are produced.
Figure 2
The staphylococcal cell envelope. The staphylococcal cell wall is composed of multilayers of glycan strands of alternating _N_‐acetylglucosamine (Glc_N_Ac) and _N_‐acetylmuramyl‐pentapeptide (Mur_N_Ac‐PP), cross‐linked by pentaglycine side‐chains. For clarity, only one layer of peptidoglycan is depicted here. Teichoic acids, polyanionic surface polymers extending through the peptidoglycan layer, contribute to the negative charge of the cell wall. The cytoplasmic membrane is a selectively permeable membrane lying internal to the cell wall.
Figure 3
Detailed structure of a chitosan molecule. Shown is an enlarged portion of a chitosan molecule, with relevant dimensions. For a chitosan molecule of a MW of 240 kDa and a DD of 87%, an average chain would consist of around 1400 units, with an average length of about 700 nm.
Similar articles
- Quaternized chitosan as an antimicrobial agent: antimicrobial activity, mechanism of action and biomedical applications in orthopedics.
Tan H, Ma R, Lin C, Liu Z, Tang T. Tan H, et al. Int J Mol Sci. 2013 Jan 16;14(1):1854-69. doi: 10.3390/ijms14011854. Int J Mol Sci. 2013. PMID: 23325051 Free PMC article. Review. - Chitin/chitosan derivatives and their interactions with microorganisms: a comprehensive review and future perspectives.
Riaz Rajoka MS, Mehwish HM, Wu Y, Zhao L, Arfat Y, Majeed K, Anwaar S. Riaz Rajoka MS, et al. Crit Rev Biotechnol. 2020 May;40(3):365-379. doi: 10.1080/07388551.2020.1713719. Epub 2020 Jan 16. Crit Rev Biotechnol. 2020. PMID: 31948287 Review. - Pharmaceutical applications of chitosan.
Shariatinia Z. Shariatinia Z. Adv Colloid Interface Sci. 2019 Jan;263:131-194. doi: 10.1016/j.cis.2018.11.008. Epub 2018 Nov 30. Adv Colloid Interface Sci. 2019. PMID: 30530176 Review. - Antimicrobial Chitosan based formulations with impact on different biomedical applications.
Radulescu M, Ficai D, Oprea O, Ficai A, Andronescu E, Holban AM. Radulescu M, et al. Curr Pharm Biotechnol. 2015;16(2):128-36. doi: 10.2174/138920101602150112151157. Curr Pharm Biotechnol. 2015. PMID: 25594289 Review. - Antimicrobial Chitosan Conjugates: Current Synthetic Strategies and Potential Applications.
Qin Y, Li P. Qin Y, et al. Int J Mol Sci. 2020 Jan 13;21(2):499. doi: 10.3390/ijms21020499. Int J Mol Sci. 2020. PMID: 31941068 Free PMC article. Review.
Cited by
- Quaternized chitosan as an antimicrobial agent: antimicrobial activity, mechanism of action and biomedical applications in orthopedics.
Tan H, Ma R, Lin C, Liu Z, Tang T. Tan H, et al. Int J Mol Sci. 2013 Jan 16;14(1):1854-69. doi: 10.3390/ijms14011854. Int J Mol Sci. 2013. PMID: 23325051 Free PMC article. Review. - AMP-Chitosan Coating with Bactericidal Activity in the Presence of Human Plasma Proteins.
Monteiro C, Fernandes H, Oliveira D, Vale N, Barbosa M, Gomes P, L Martins MC. Monteiro C, et al. Molecules. 2020 Jul 3;25(13):3046. doi: 10.3390/molecules25133046. Molecules. 2020. PMID: 32635294 Free PMC article. - Computational Assessment of Chito-Oligosaccharides Interactions with Plasma Proteins.
Roman DL, Ostafe V, Isvoran A. Roman DL, et al. Mar Drugs. 2021 Feb 24;19(3):120. doi: 10.3390/md19030120. Mar Drugs. 2021. PMID: 33668290 Free PMC article. - Preparation, Structural Characterization, and Enzymatic Properties of Alginate Lyase Immobilized on Magnetic Chitosan Microspheres.
Li J, Yan F, Huang B, Zhang M, Wu X, Liu Y, Ruan R, Zheng H. Li J, et al. Appl Biochem Biotechnol. 2024 Aug;196(8):5403-5418. doi: 10.1007/s12010-023-04824-z. Epub 2023 Dec 30. Appl Biochem Biotechnol. 2024. PMID: 38158490 - Chitin and chitosan as tools to combat COVID-19: A triple approach.
Safarzadeh M, Sadeghi S, Azizi M, Rastegari-Pouyani M, Pouriran R, Haji Molla Hoseini M. Safarzadeh M, et al. Int J Biol Macromol. 2021 Jul 31;183:235-244. doi: 10.1016/j.ijbiomac.2021.04.157. Epub 2021 Apr 27. Int J Biol Macromol. 2021. PMID: 33930442 Free PMC article. Review.
References
- Anandan R., Nair P.G.V., Mathew S. Anti‐ulcerogenic effect of chitin and chitosan on mucosal antioxidant defence system in HCl‐ethanol‐induced ulcer in rats. J Pharm Pharmacol. 2004;56:265–269. - PubMed
- Babel S., Kurniawan T.A. Low‐cost adsorbents for heavy metals uptake from contaminated water: a review. J Hazard Mater. 2003;97:219–243. - PubMed
- Beauséjour J., Clermont N., Beaulieu C. Effect of Streptomyces melanosporofaciens strain EF‐76 and of chitosan on common scab of potato. Plant Soil. 2003;256:463–468.
- Bernkop‐Schnürch A. Chitosan and its derivatives: potential excipients for peroral peptide delivery systems. Int J Pharm. 2000;194:1–13. - PubMed
- Bhatia S.C., Ravi N. A Mössbauer study of the interaction of chitosan and d‐glucosamine with iron and its relevance to other metalloenzymes. Biomacromolecules. 2003;4:723–727. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources