Roles of reactive oxygen and nitrogen species in pain - PubMed (original) (raw)
Review
Roles of reactive oxygen and nitrogen species in pain
Daniela Salvemini et al. Free Radic Biol Med. 2011.
Abstract
Peroxynitrite (PN; ONOO⁻) and its reactive oxygen precursor superoxide (SO; O₂•⁻) are critically important in the development of pain of several etiologies including pain associated with chronic use of opiates such as morphine (also known as opiate-induced hyperalgesia and antinociceptive tolerance). This is now an emerging field in which considerable progress has been made in terms of understanding the relative contributions of SO, PN, and nitroxidative stress in pain signaling at the molecular and biochemical levels. Aggressive research in this area is poised to provide the pharmacological basis for development of novel nonnarcotic analgesics that are based upon the unique ability to selectively eliminate SO and/or PN. As we have a better understanding of the roles of SO and PN in pathophysiological settings, targeting PN may be a better therapeutic strategy than targeting SO. This is because, unlike PN, which has no currently known beneficial role, SO may play a significant role in learning and memory. Thus, the best approach may be to spare SO while directly targeting its downstream product, PN. Over the past 15 years, our team has spearheaded research concerning the roles of SO and PN in pain and these results are currently leading to the development of solid therapeutic strategies in this important area.
Copyright © 2011 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
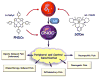
Fig. 1. Superoxide and peroxynitrite are targets for novel pain therapy
Superoxide (O2·−) and peroxynitrite (ONOO−) are key mediators in the development of peripheral and central sensitization of the various pain etiologies. The use of superoxide-dismutase mimetics (SODm, i.e. SC-72325) and peroxynitrite decomposition catalysts (PNDCs, i.e. FeTMPyP) reduce nitroxidative stress and attenuate the development of peripheral and central sensitization; providing promising novel therapy for chronic pain management.
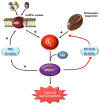
Fig. 2. Peroxynitrite-reinforced superoxide production in central sensitization: two feed forward mechanisms
Two major sites of superoxide (O2·−) production, NADPH oxidase and mitochondrial respiration, are active in the development of central sensitization. Peroxynitrite (ONOO−) formed from NADPH oxidase- and mitochondrial-derived superoxide nitrates and inactivates the manganese SOD (MnSOD) enzyme preventing the removal of mitochondrial-derived superoxide. Peroxynitrite enhances protein kinase C (PKC) activity and, in turn, enhances translocation of NADPH oxidase regulatory subunits to the membrane to increase the NADPH oxidase-derived superoxide production. Combined, these two mechanisms amplify superoxide-derived peroxynitrite formation leading to the development of central sensitization.
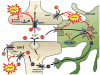
Fig. 3. The role of peroxynitrite in glutamatergic homeostasis and signaling
Peroxynitrite (ONOO−) enhances glutamatergic signaling through nitration and activation of NMDARs and the protein kinases responsible for NMDAR activation. Peroxynitrite further enhances glutamatergic signaling by nitrating and inactivating the glutamate transporters (GLT-1, GLAST, and EAAC1) that remove glutamate (Glu) from the synapse and extrasynaptic regions and glutamine synthetase (GS) that converts glutamate, ammonia and ATP to glutamine, which is then taken back up by the neurons via the glutamine transporter. Nitration and inactivation of these enzymes results in toxic levels of glutamate and activation of neuroimmune responses. Inactivation of GS may also lead to increase ammonia levels that can inhibit glutamate transport. In addition to glutamate uptake, EAAC1 transports cysteine (Cys) into the neuron a process that is key in the biosynthesis of glutathione (GSH), a major cellular antioxidant. Compromised Cys transport by nitrated EAAC1 could lead to increased neuronal nitroxidative stress.
Similar articles
- Targeting peroxynitrite driven nitroxidative stress with synzymes: A novel therapeutic approach in chronic pain management.
Salvemini D, Neumann W. Salvemini D, et al. Life Sci. 2010 Apr 10;86(15-16):604-14. doi: 10.1016/j.lfs.2009.06.011. Epub 2009 Jul 1. Life Sci. 2010. PMID: 19576230 Review. - Reactive nitroxidative species and nociceptive processing: determining the roles for nitric oxide, superoxide, and peroxynitrite in pain.
Little JW, Doyle T, Salvemini D. Little JW, et al. Amino Acids. 2012 Jan;42(1):75-94. doi: 10.1007/s00726-010-0633-0. Epub 2010 Jun 16. Amino Acids. 2012. PMID: 20552384 Review. - Supraspinal inactivation of mitochondrial superoxide dismutase is a source of peroxynitrite in the development of morphine antinociceptive tolerance.
Doyle T, Bryant L, Batinic-Haberle I, Little J, Cuzzocrea S, Masini E, Spasojevic I, Salvemini D. Doyle T, et al. Neuroscience. 2009 Dec 1;164(2):702-10. doi: 10.1016/j.neuroscience.2009.07.019. Epub 2009 Jul 14. Neuroscience. 2009. PMID: 19607887 Free PMC article. - Anti-superoxide and anti-peroxynitrite strategies in pain suppression.
Janes K, Neumann WL, Salvemini D. Janes K, et al. Biochim Biophys Acta. 2012 May;1822(5):815-21. doi: 10.1016/j.bbadis.2011.12.008. Epub 2011 Dec 19. Biochim Biophys Acta. 2012. PMID: 22200449 Free PMC article. - Lipophilicity is a critical parameter that dominates the efficacy of metalloporphyrins in blocking the development of morphine antinociceptive tolerance through peroxynitrite-mediated pathways.
Batinić-Haberle I, Ndengele MM, Cuzzocrea S, Rebouças JS, Spasojević I, Salvemini D. Batinić-Haberle I, et al. Free Radic Biol Med. 2009 Jan 15;46(2):212-9. doi: 10.1016/j.freeradbiomed.2008.09.037. Epub 2008 Oct 17. Free Radic Biol Med. 2009. PMID: 18983908 Free PMC article.
Cited by
- PEA-OXA Mitigates Oxaliplatin-Induced Painful Neuropathy through NF-κB/Nrf-2 Axis.
Campolo M, Lanza M, Paterniti I, Filippone A, Ardizzone A, Casili G, Scuderi SA, Puglisi C, Mare M, Memeo L, Cuzzocrea S, Esposito E. Campolo M, et al. Int J Mol Sci. 2021 Apr 10;22(8):3927. doi: 10.3390/ijms22083927. Int J Mol Sci. 2021. PMID: 33920318 Free PMC article. - Endoplasmic reticulum-mitochondria interplay in chronic pain: The calcium connection.
Yousuf MS, Maguire AD, Simmen T, Kerr BJ. Yousuf MS, et al. Mol Pain. 2020 Jan-Dec;16:1744806920946889. doi: 10.1177/1744806920946889. Mol Pain. 2020. PMID: 32787562 Free PMC article. Review. - The antioxidant N-(2-mercaptopropionyl)-glycine (tiopronin) attenuates expression of neuropathic allodynia and hyperalgesia.
Shahid M, Subhan F, Islam NU, Ahmad N, Farooq U, Abbas S, Akbar S, Ullah I, Raziq N, Din ZU. Shahid M, et al. Naunyn Schmiedebergs Arch Pharmacol. 2021 Apr;394(4):603-617. doi: 10.1007/s00210-020-01995-y. Epub 2020 Oct 20. Naunyn Schmiedebergs Arch Pharmacol. 2021. PMID: 33079239 - Bioenergetic deficits in peripheral nerve sensory axons during chemotherapy-induced neuropathic pain resulting from peroxynitrite-mediated post-translational nitration of mitochondrial superoxide dismutase.
Janes K, Doyle T, Bryant L, Esposito E, Cuzzocrea S, Ryerse J, Bennett GJ, Salvemini D. Janes K, et al. Pain. 2013 Nov;154(11):2432-2440. doi: 10.1016/j.pain.2013.07.032. Epub 2013 Jul 25. Pain. 2013. PMID: 23891899 Free PMC article. - Cancer and orofacial pain.
Romero-Reyes M, Salvemini D. Romero-Reyes M, et al. Med Oral Patol Oral Cir Bucal. 2016 Nov 1;21(6):e665-e671. doi: 10.4317/medoral.21515. Med Oral Patol Oral Cir Bucal. 2016. PMID: 27694791 Free PMC article. Review.
References
- Renfrey S, Downton C, Featherstone J. The painful reality. Nat Rev Drug Discov. 2003;2:175–176. - PubMed
- Mao J, Price DD, Mayer DJ. Mechanisms of hyperalgesia and morphine tolerance: a current view of their possible interactions. Pain. 1995;62:259–274. - PubMed
- Arner S, Rawal N, Gustafsson LL. Clinical experience of long-term treatment with epidural and intrathecal opioids--a nationwide survey. Acta Anaesthesiol Scand. 1988;32:253–259. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous