Antibiotic treatment alters the colonic mucus layer and predisposes the host to exacerbated Citrobacter rodentium-induced colitis - PubMed (original) (raw)
Antibiotic treatment alters the colonic mucus layer and predisposes the host to exacerbated Citrobacter rodentium-induced colitis
M Wlodarska et al. Infect Immun. 2011 Apr.
Abstract
Antibiotics are often used in the clinic to treat bacterial infections, but the effects of these drugs on microbiota composition and on intestinal immunity are poorly understood. Citrobacter rodentium was used as a model enteric pathogen to investigate the effect of microbial perturbation on intestinal barriers and susceptibility to colitis. Streptomycin and metronidazole were used to induce alterations in the composition of the microbiota prior to infection with C. rodentium. Metronidazole pretreatment increased susceptibility to C. rodentium-induced colitis over that of untreated and streptomycin-pretreated mice, 6 days postinfection. Both antibiotic treatments altered microbial composition, without affecting total numbers, but metronidazole treatment resulted in a more dramatic change, including a reduced population of Porphyromonadaceae and increased numbers of lactobacilli. Disruption of the microbiota with metronidazole, but not streptomycin treatment, resulted in an increased inflammatory tone of the intestine characterized by increased bacterial stimulation of the epithelium, altered goblet cell function, and thinning of the inner mucus layer, suggesting a weakened mucosal barrier. This reduction in mucus thickness correlates with increased attachment of C. rodentium to the intestinal epithelium, contributing to the exacerbated severity of C. rodentium-induced colitis in metronidazole-pretreated mice. These results suggest that antibiotic perturbation of the microbiota can disrupt intestinal homeostasis and the integrity of intestinal defenses, which protect against invading pathogens and intestinal inflammation.
Figures
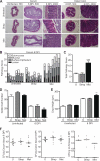
FIG. 1.
Metronidazole pretreatment leads to increased severity of _C. rodentium_-induced colitis. (A) H&E-stained cecal and distal colon sections from untreated and streptomycin- and metronidazole-pretreated mice at day 0 and 6 days p.i. No inflammation is evident in cecal sections of untreated and antibiotic-pretreated mice prior to infection (left panels; original magnification, ×50; bars, 100 μm). Notably, there is an increase in inflammation throughout the cecal mucosa and submucosa of metronidazole-pretreated mice (original magnification, ×50; bars, 100 μm). Goblet cell depletion and hyperplasia are more extensive in the mucosa of metronidazole-pretreated mice (black arrowhead, goblet cell; right panels; original magnification, ×400) both in the cecum and in the distal colon. (B) Independent histology damage scores from cecal tissues of untreated, streptomycin-pretreated, and metronidazole-pretreated mice at day 6 p.i. Scores were determined under blinded conditions. Each bar represents one individual based on inflammation and damage to the submucosa, mucosa, surface epithelium, and lumen. (C) Cumulative histology damage scores from cecal tissue at day 6 p.i. of untreated, streptomycin-pretreated, and metronidazole-pretreated mice. Scores were determined under blinded conditions. Cecal results represent the means of two independent infections (n = 4 per group). U, untreated; ***, P = 0.0003. (D) Goblet cell numbers of untreated, streptomycin-pretreated, and metronidazole-pretreated mice. Goblet cell numbers prior to infection and at day 6 p.i. in the distal colon are shown. Results represent the means of two independent experiments (n = 4 per group). U, untreated; **, P = 0.0011. (E) Hyperplasia was measured as changes in crypt length in untreated, streptomycin-pretreated, and metronidazole-pretreated mice. Hyperplasia prior to infection and that at day 6 p.i. in the distal colon are shown. Results represent the means of two independent experiments (n = 4 per group). U, untreated; **, P = 0.0044. (F) Enumeration of C. rodentium bacteria in the cecum, colon, and mesenteric lymph node tissue at day 6 p.i. Each data point represents one individual. Results are pooled from three separate infections (n = 4 to 6 per group). The horizontal lines represent the median for each group. U, untreated.
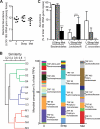
FIG. 2.
Metronidazole treatment and streptomycin treatment of mice differentially alter the microbial composition of the colon compared to that of untreated mice. (A) Total bacterial numbers determined by real-time PCR measuring Eubacteria 16S rRNA in feces of untreated (U) and streptomycin- and metronidazole-treated mice. Results are averaged from two independent experiments (n = 3 or 4 mice per group). (B) Similarity tree using Bray-Curtis metrics of bacterial 16S rRNA gene terminal restriction fragment profiles from distal colon samples. Bar graphs represent average T-RFLP profiles from each treatment group, and the bacterial families represented by terminal restriction fragment lengths (TRFs; cut with MspI) of interest are indicated. U, untreated mice; S, streptomycin-treated mice; M, metronidazole-treated mice. Results are representative of two independent experiments (n = 3 or 4 mice per group). (C) Real-time PCR quantification of select bacterial populations using group-specific primers on DNA extracted from distal colon samples. The abundance of target groups was normalized to the total bacterial 16S rRNA gene copies in each sample. Results are averaged from two independent experiments (n = 3 or 4 mice per group). ***, P < 0.0001.
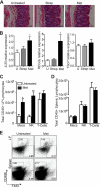
FIG. 3.
Metronidazole treatment alters the homeostatic balance of the distal colon. (A) H&E-stained distal colon sections from untreated and streptomycin- and metronidazole-treated mice at day 0. No overt inflammation evident. Original magnification, ×400; bars, 50 μm. (B) Quantitative RT-PCR results of IL-25 (*, P = 0.0371), _Reg3_γ (*, P = 0.0177); and TSLP expression in the distal colon of untreated and streptomycin- and metronidazole-treated mice. Results are averaged from three independent experiments (n = 4 to 6 mice per group). U, untreated mice. (C and D) FACS analysis of macrophage, NK cell, and T cell recruitment to the lamina propria (C) and spleen (D) in untreated and metronidazole-treated mice. Both splenic and lamina propria cells were stained with fluorescently labeled CD45, CD3ɛ, CD49b, and F480. Only CD45-positive cells were examined for the expression of CD3ɛ, CD49b, and F480. T cells are defined as CD45+ CD3ɛ+ CD49b−; NK cells are defined as CD45+ CD3ɛ− CD49b+; macrophages are defined as CD45+ F480+. (E) Flow cytometric analysis of CD49b+ and F480+ populations within the CD45+ lymphocytes in the lamina propria and spleen of untreated and metronidazole-treated mice. Data from one mouse per group are shown and are representative of 2 independent experiments (n = 4 per group).
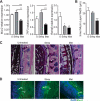
FIG. 4.
Metronidazole treatment alters goblet cell function and reduces the production of the inner mucus layer. (A) Quantitative RT-PCR results of Muc2, TFF3, and _Relm_β expression in the distal colon. Results are averaged from three independent experiments (n = 3 to 6 mice per group). U, untreated mice; for Muc2, ***, P = 0.0008, and **, P = 0.0017; for TFF3, ***, P < 0.0001, and *, P = 0.02; for _Relm_β, Strep *, P = 0.03, and Met *, P = 0.01. (B) Quantification of inner mucin layer thickness. Distal colon sections were fixed in methanol-Carnoy's fixative, embedded in paraffin, and stained with AB/PAS to visualize and quantify the inner mucus layer. The inner mucus width was determined by an average of 4 measurements per field with 4 fields counted per tissue section. Results are averaged from two independent experiments (n = 3 mice per group). U, untreated mice; ***, P = 0.0003. (C) AB/PAS-stained methanol-Carnoy's fixative-fixed distal colon sections showing the inner mucin layer (white arrowheads). i, inner mucin layer; GC, goblet cell. Original magnification, ×400. Bars, 20 μm. (D) Representative immunostaining for the inner mucin layer using an antibody that recognizes murine Muc2 (green) with DAPI (blue) as a counterstain. The inner mucin layer is thinner in metronidazole-treated C57BL/6 mice. i, inner mucin layer; GC, goblet cell. Original magnification, ×400. Bars, 20 μm.
FIG. 5.
Metronidazole pretreatment increases the rate of C. rodentium attachment to intestinal epithelial cells. (A) Representative immunostaining for the _C. rodentium_-specific effector Tir (green) in colon, with DAPI (blue) as a counterstain, at various time points for untreated and metronidazole-pretreated mice. Note Tir staining present at day 2 p.i. in metronidazole-pretreated mice, which is absent in untreated mice. C. rodentium penetrates deeper into crypts of metronidazole-pretreated mice than it does into those of untreated mice on day 4 p.i. (asterisk). Original magnification, ×100. Bars, 100 μm. (B) Enumeration of C. rodentium bacteria in the colon at days 2, 4, 6, and 21 p.i. (2 DPI, 4 DPI, 6 DPI, and 21 DPI, respectively). Each data point represents one individual. Results are pooled from two separate infections (n = 4 to 6 per group). **, P = 0.0068. Day 21 p.i. results represent a single experiment (n = 6). The horizontal lines represent the median for each group. U, untreated. (C) Cytokine and chemokine production in the colon at days 2, 4, and 6 p.i. (2 DPI, 4 DPI, and 6 DPI, respectively). Results are pooled from two separate infections (n = 4 to 6 per group). Cytokines TNF-α and IFN-γ and chemokine MCP-1 are produced at 6-fold (***, P = 0.0007)-, 5-fold (**, P = 0.0033)-, and 4-fold (*, P = 0.0343)-higher levels, respectively, at day 2 p.i. in metronidazole-pretreated mice than in untreated mice.
Similar articles
- Effects of hypoxic exposure on immune responses of intestinal mucosa to Citrobacter colitis in mice.
Ji Q, Zhang Y, Zhou Y, Gamah M, Yuan Z, Liu J, Cao C, Gao X, Zhang H, Ren Y, Zhang W. Ji Q, et al. Biomed Pharmacother. 2020 Sep;129:110477. doi: 10.1016/j.biopha.2020.110477. Epub 2020 Jul 6. Biomed Pharmacother. 2020. PMID: 32768962 - Citrobacter rodentium-induced colitis: A robust model to study mucosal immune responses in the gut.
Koroleva EP, Halperin S, Gubernatorova EO, Macho-Fernandez E, Spencer CM, Tumanov AV. Koroleva EP, et al. J Immunol Methods. 2015 Jun;421:61-72. doi: 10.1016/j.jim.2015.02.003. Epub 2015 Feb 19. J Immunol Methods. 2015. PMID: 25702536 - Concurrent infection with an intestinal helminth parasite impairs host resistance to enteric Citrobacter rodentium and enhances Citrobacter-induced colitis in mice.
Chen CC, Louie S, McCormick B, Walker WA, Shi HN. Chen CC, et al. Infect Immun. 2005 Sep;73(9):5468-81. doi: 10.1128/IAI.73.9.5468-5481.2005. Infect Immun. 2005. PMID: 16113263 Free PMC article. - Citrobacter rodentium: infection, inflammation and the microbiota.
Collins JW, Keeney KM, Crepin VF, Rathinam VA, Fitzgerald KA, Finlay BB, Frankel G. Collins JW, et al. Nat Rev Microbiol. 2014 Sep;12(9):612-23. doi: 10.1038/nrmicro3315. Epub 2014 Aug 4. Nat Rev Microbiol. 2014. PMID: 25088150 Review. - Overview of the Effect of Citrobacter rodentium Infection on Host Metabolism and the Microbiota.
Hopkins EGD, Frankel G. Hopkins EGD, et al. Methods Mol Biol. 2021;2291:399-418. doi: 10.1007/978-1-0716-1339-9_20. Methods Mol Biol. 2021. PMID: 33704766 Review.
Cited by
- A single early-in-life antibiotic course increases susceptibility to DSS-induced colitis.
Ozkul C, Ruiz VE, Battaglia T, Xu J, Roubaud-Baudron C, Cadwell K, Perez-Perez GI, Blaser MJ. Ozkul C, et al. Genome Med. 2020 Jul 25;12(1):65. doi: 10.1186/s13073-020-00764-z. Genome Med. 2020. PMID: 32711559 Free PMC article. - When pathogenic bacteria meet the intestinal microbiota.
Rolhion N, Chassaing B. Rolhion N, et al. Philos Trans R Soc Lond B Biol Sci. 2016 Nov 5;371(1707):20150504. doi: 10.1098/rstb.2015.0504. Philos Trans R Soc Lond B Biol Sci. 2016. PMID: 27672153 Free PMC article. Review. - Citrobacter rodentium possesses a functional type II secretion system necessary for successful host infection.
Krekhno Z, Woodward SE, Serapio-Palacios A, Peña-Díaz J, Moon KM, Foster LJ, Finlay BB. Krekhno Z, et al. Gut Microbes. 2024 Jan-Dec;16(1):2308049. doi: 10.1080/19490976.2024.2308049. Epub 2024 Feb 1. Gut Microbes. 2024. PMID: 38299318 Free PMC article. - Neonatal Exposure to Amoxicillin Alters Long-Term Immune Response Despite Transient Effects on Gut-Microbiota in Piglets.
Fouhse JM, Yang K, More-Bayona J, Gao Y, Goruk S, Plastow G, Field CJ, Barreda DR, Willing BP. Fouhse JM, et al. Front Immunol. 2019 Sep 4;10:2059. doi: 10.3389/fimmu.2019.02059. eCollection 2019. Front Immunol. 2019. PMID: 31552023 Free PMC article. - The Nlrp6 inflammasome is not required for baseline colonic inner mucus layer formation or function.
Volk JK, Nyström EEL, van der Post S, Abad BM, Schroeder BO, Johansson Å, Svensson F, Jäverfelt S, Johansson MEV, Hansson GC, Birchenough GMH. Volk JK, et al. J Exp Med. 2019 Nov 4;216(11):2602-2618. doi: 10.1084/jem.20190679. Epub 2019 Aug 16. J Exp Med. 2019. PMID: 31420376 Free PMC article.
References
- Ambrose, N. S., et al. 1985. Antibiotic therapy for treatment in relapse of intestinal Crohn's disease. A prospective randomized study. Dis. Colon Rectum 28:81-85. - PubMed
- Atuma, C., V. Strugala, A. Allen, and L. Holm. 2001. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am. J. Physiol. Gastrointest. Liver Physiol. 280:G922-G929. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical