Four faces of cellular senescence - PubMed (original) (raw)
Review
Four faces of cellular senescence
Francis Rodier et al. J Cell Biol. 2011.
Abstract
Cellular senescence is an important mechanism for preventing the proliferation of potential cancer cells. Recently, however, it has become apparent that this process entails more than a simple cessation of cell growth. In addition to suppressing tumorigenesis, cellular senescence might also promote tissue repair and fuel inflammation associated with aging and cancer progression. Thus, cellular senescence might participate in four complex biological processes (tumor suppression, tumor promotion, aging, and tissue repair), some of which have apparently opposing effects. The challenge now is to understand the senescence response well enough to harness its benefits while suppressing its drawbacks.
Figures
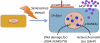
Figure 1.
Hallmarks of senescent cells. Senescent cells differ from other nondividing (quiescent, terminally differentiated) cells in several ways, although no single feature of the senescent phenotype is exclusively specific. Hallmarks of senescent cells include an essentially irreversible growth arrest; expression of SA-Bgal and p16INK4a; robust secretion of numerous growth factors, cytokines, proteases, and other proteins (SASP); and nuclear foci containing DDR proteins (DNA-SCARS/TIF) or heterochromatin (SAHF). The pink circles in the nonsenescent cell (left) and senescent cell (right) represent the nucleus.
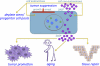
Figure 2.
Biological activities of cellular senescence. Senescent cells arrest growth owing to cell autonomous mechanisms, imposed by the p53 and p16INK4a/pRB tumor suppressor pathways, and cell nonautonomous mechanisms, imposed by some of the proteins that comprise the SASP. The growth arrest is the main feature by which cellular senescence suppresses malignant tumorigenesis but can contribute to the depletion of proliferative (stem/progenitor) cell pools. Additionally, components of the SASP can promote tumor progression, facilitate wound healing, and, possibly, contribute to aging.
Figure 3.
Temporal organization of the senescent phenotype. Upon experiencing a potentially oncogenic insult, cells assess the stress and must “decide” whether to attempt repair and recovery, or undergo senescence. After an interval (decision period), the length of which is imprecisely known, the senescence growth arrest becomes essentially permanent, effectively suppressing the ability of the stressed cell to form a malignant tumor. One early manifestation of the senescent phenotype is the expression of cell surface–bound IL-1α. This cytokine acts in a juxtacrine manner to bind the cell surface–bound IL-1 receptor, which initiates a signaling cascade that activates transcription factors (NF-κB, C/EBPβ). The transcription factors subsequently stimulate the expression of many secreted (SASP) proteins, including increasing the expression of IL-1α and inducing expression of the inflammatory cytokines IL-6 and IL-8. These positive cytokine feedback loops intensify the SASP until it reaches levels found in senescent cells. SASP components such as IL-6, IL-8, and MMPs can promote tissue repair, but also cancer progression. Some SASP proteins, in conjunction with cell surface ligands and adhesion molecules expressed by senescent cells, eventually attract immune cells that kill and clear senescent cells. A late manifestation of the senescent phenotype is the expression of microRNAs (mir-146a and mir-146b), which tune down the expression IL-6, IL-8, and possibly other SASP proteins, presumably to prevent the SASP from generating a persistent acute inflammatory response. Despite this dampening effect, the SASP can nonetheless continue to generate low-level chronic inflammation. The accumulation of senescent cells that either escape or outpace immune clearance and express a SASP at chronic low levels is hypothesized to drive aging phenotypes. Thus, senescent cells, over time (yellow line), develop a phenotype that becomes increasingly complex (blue triangle), with both beneficial (tumor suppression and tissue repair) and deleterious (tumor promotion and aging) effects on the health of the organism.
Similar articles
- On the evolution of cellular senescence.
Kowald A, Passos JF, Kirkwood TBL. Kowald A, et al. Aging Cell. 2020 Dec;19(12):e13270. doi: 10.1111/acel.13270. Epub 2020 Nov 9. Aging Cell. 2020. PMID: 33166065 Free PMC article. Review. - Cellular senescence: a link between cancer and age-related degenerative disease?
Campisi J, Andersen JK, Kapahi P, Melov S. Campisi J, et al. Semin Cancer Biol. 2011 Dec;21(6):354-9. doi: 10.1016/j.semcancer.2011.09.001. Epub 2011 Sep 10. Semin Cancer Biol. 2011. PMID: 21925603 Free PMC article. Review. - Cellular senescence: putting the paradoxes in perspective.
Campisi J. Campisi J. Curr Opin Genet Dev. 2011 Feb;21(1):107-12. doi: 10.1016/j.gde.2010.10.005. Epub 2010 Nov 17. Curr Opin Genet Dev. 2011. PMID: 21093253 Free PMC article. Review. - Senescence under appraisal: hopes and challenges revisited.
Davan-Wetton CSA, Pessolano E, Perretti M, Montero-Melendez T. Davan-Wetton CSA, et al. Cell Mol Life Sci. 2021 Apr;78(7):3333-3354. doi: 10.1007/s00018-020-03746-x. Epub 2021 Jan 13. Cell Mol Life Sci. 2021. PMID: 33439271 Free PMC article. Review. - Premature aging/senescence in cancer cells facing therapy: good or bad?
Gonzalez LC, Ghadaouia S, Martinez A, Rodier F. Gonzalez LC, et al. Biogerontology. 2016 Feb;17(1):71-87. doi: 10.1007/s10522-015-9593-9. Epub 2015 Sep 2. Biogerontology. 2016. PMID: 26330289 Review.
Cited by
- Construction of a computable network model for DNA damage, autophagy, cell death, and senescence.
Gebel S, Lichtner RB, Frushour B, Schlage WK, Hoang V, Talikka M, Hengstermann A, Mathis C, Veljkovic E, Peck M, Peitsch MC, Deehan R, Hoeng J, Westra JW. Gebel S, et al. Bioinform Biol Insights. 2013;7:97-117. doi: 10.4137/BBI.S11154. Epub 2013 Mar 7. Bioinform Biol Insights. 2013. PMID: 23515068 Free PMC article. - Genetic differences in transcript responses to low-dose ionizing radiation identify tissue functions associated with breast cancer susceptibility.
Snijders AM, Marchetti F, Bhatnagar S, Duru N, Han J, Hu Z, Mao JH, Gray JW, Wyrobek AJ. Snijders AM, et al. PLoS One. 2012;7(10):e45394. doi: 10.1371/journal.pone.0045394. Epub 2012 Oct 15. PLoS One. 2012. PMID: 23077491 Free PMC article. - Beyond DNA repair and chromosome instability-Fanconi anaemia as a cellular senescence-associated syndrome.
Helbling-Leclerc A, Garcin C, Rosselli F. Helbling-Leclerc A, et al. Cell Death Differ. 2021 Apr;28(4):1159-1173. doi: 10.1038/s41418-021-00764-5. Epub 2021 Mar 15. Cell Death Differ. 2021. PMID: 33723374 Free PMC article. Review. - m6A-independent genome-wide METTL3 and METTL14 redistribution drives the senescence-associated secretory phenotype.
Liu P, Li F, Lin J, Fukumoto T, Nacarelli T, Hao X, Kossenkov AV, Simon MC, Zhang R. Liu P, et al. Nat Cell Biol. 2021 Apr;23(4):355-365. doi: 10.1038/s41556-021-00656-3. Epub 2021 Apr 1. Nat Cell Biol. 2021. PMID: 33795874 Free PMC article. - Conserved Senescence Associated Genes and Pathways in Primary Human Fibroblasts Detected by RNA-Seq.
Marthandan S, Baumgart M, Priebe S, Groth M, Schaer J, Kaether C, Guthke R, Cellerino A, Platzer M, Diekmann S, Hemmerich P. Marthandan S, et al. PLoS One. 2016 May 3;11(5):e0154531. doi: 10.1371/journal.pone.0154531. eCollection 2016. PLoS One. 2016. PMID: 27140416 Free PMC article.
References
- Alimonti A., Nardella C., Chen Z., Clohessy J.G., Carracedo A., Trotman L.C., Cheng K., Varmeh S., Kozma S.C., Thomas G., et al. 2010. A novel type of cellular senescence that can be enhanced in mouse models and human tumor xenografts to suppress prostate tumorigenesis. J. Clin. Invest. 120:681–693 10.1172/JCI40535 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources