Assessment of hypermucoviscosity as a virulence factor for experimental Klebsiella pneumoniae infections: comparative virulence analysis with hypermucoviscosity-negative strain - PubMed (original) (raw)
Comparative Study
Assessment of hypermucoviscosity as a virulence factor for experimental Klebsiella pneumoniae infections: comparative virulence analysis with hypermucoviscosity-negative strain
Yi-Chun Lin et al. BMC Microbiol. 2011.
Abstract
Background: Klebsiella pneumoniae displaying the hypermucoviscosity (HV) phenotype are considered more virulent than HV-negative strains. Nevertheless, the emergence of tissue-abscesses-associated HV-negative isolates motivated us to re-evaluate the role of HV-phenotype.
Results: Instead of genetically manipulating the HV-phenotype of K. pneumoniae, we selected two clinically isolated K1 strains, 1112 (HV-positive) and 1084 (HV-negative), to avoid possible interference from defects in the capsule. These well-encapsulated strains with similar genetic backgrounds were used for comparative analysis of bacterial virulence in a pneumoniae or a liver abscess model generated in either naïve or diabetic mice. In the pneumonia model, the HV-positive strain 1112 proliferated to higher loads in the lungs and blood of naïve mice, but was less prone to disseminate into the blood of diabetic mice compared to the HV-negative strain 1084. In the liver abscess model, 1084 was as potent as 1112 in inducing liver abscesses in both the naïve and diabetic mice. The 1084-infected diabetic mice were more inclined to develop bacteremia and had a higher mortality rate than those infected by 1112. A mini-Tn5 mutant of 1112, isolated due to its loss of HV-phenotype, was avirulent to mice.
Conclusion: These results indicate that the HV-phenotype is required for the virulence of the clinically isolated HV-positive strain 1112. The superior ability of the HV-negative stain 1084 over 1112 to cause bacteremia in diabetic mice suggests that factors other than the HV phenotype were required for the systemic dissemination of K. pneumoniae in an immunocompromised setting.
Figures
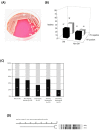
Figure 1
Prevalence of HV phenotype among clinical K. pneumoniae isolates. (A) A mucoviscous string formed between an inoculation loop and the colony of a HV-positive strain. (B) Occurrence of HV-positive (black columns) or HV-negative (white columns) isolates in patients with or without diabetic mellitus (DM or Non-DM). (C) Prevalence of HV-positive K. pneumoniae among patients suffering from various infections, including KLA, non-hepatic abscess, pneumonia, primary bacteremia, and secondary bacteremia. (D) Dendrogram of the HV-positive strain 1112 and-negative strain 1084. Genetic similarities were calculated using UPGMA.
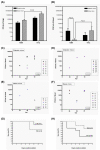
Figure 2
Analysis of comparative virulence analysis for HV-positive and -negative K. pneumoniae. In the pneumonia model, bacterial counts in the lung (A) and blood (B) at 20 hours post-infection with the HV-negative 1084 or the HV-positive 1112 were determined in diabetic mice (filled columns) or naïve mice (striped columns). In the KLA model, 1084 (C, E) and 1112 (D, F) were orally inoculated into diabetic mice with inoculums of 105 CFU (C, D) or into naïve mice with inoculums of 108 CFU (E, F). Twenty microliters of blood was removed from the retroorbital sinus of mice at 24 h, 48 h, and 72 h post-inoculation; and the bacterial loads were determined using the plate-counting method. Each symbol represents the data obtained from a particular mouse. The bacterial load recovered from a particular mouse tissue, which was beyond the detection limit (approximately 40 CFU), is not represented. Survival of these mice was monitored daily for seven days. The survival rate of the 1112-infected (solid line) or the 1084-infected (dotted line) diabetic (G) or naïve (H) mice was determined by Kaplan-Meier analysis. Data were compiled from results obtained from three independent experiments.
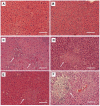
Figure 3
Histopathological examination of livers. Mice that had been orally inoculated with PBS (A, B), HV-negative strain 1084 (C, D), or HV-positive strain 1112 (E, F) (in diabetic mice) (A, C, E) with inoculums of 105 CFU or in naive mice with inoculums of 108 CFU (B, D, F) were euthanized at seven days post-inoculation. Arrows indicate the area of PMN infiltration and aggregation (100 × magnification). Scale bar represents a distance of 1 μm.
Similar articles
- Clinical implications of hypermucoviscosity phenotype in Klebsiella pneumoniae isolates: association with invasive syndrome in patients with community-acquired bacteraemia.
Lee HC, Chuang YC, Yu WL, Lee NY, Chang CM, Ko NY, Wang LR, Ko WC. Lee HC, et al. J Intern Med. 2006 Jun;259(6):606-14. doi: 10.1111/j.1365-2796.2006.01641.x. J Intern Med. 2006. PMID: 16704562 - Clinical and molecular characteristics of Klebsiella pneumoniae ventilator-associated pneumonia in mainland China.
Guo S, Xu J, Wei Y, Xu J, Li Y, Xue R. Guo S, et al. BMC Infect Dis. 2016 Oct 26;16(1):608. doi: 10.1186/s12879-016-1942-z. BMC Infect Dis. 2016. PMID: 27782809 Free PMC article. - Microbiological and clinical characteristics of bacteraemia caused by the hypermucoviscosity phenotype of Klebsiella pneumoniae in Korea.
Jung SW, Chae HJ, Park YJ, Yu JK, Kim SY, Lee HK, Lee JH, Kahng JM, Lee SO, Lee MK, Lim JH, Lee CH, Chang SJ, Ahn JY, Lee JW, Park YG. Jung SW, et al. Epidemiol Infect. 2013 Feb;141(2):334-40. doi: 10.1017/S0950268812000933. Epub 2012 May 14. Epidemiol Infect. 2013. PMID: 22578630 Free PMC article. - Antimicrobial Resistance of Hypervirulent Klebsiella pneumoniae: Epidemiology, Hypervirulence-Associated Determinants, and Resistance Mechanisms.
Lee CR, Lee JH, Park KS, Jeon JH, Kim YB, Cha CJ, Jeong BC, Lee SH. Lee CR, et al. Front Cell Infect Microbiol. 2017 Nov 21;7:483. doi: 10.3389/fcimb.2017.00483. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 29209595 Free PMC article. Review. - Hypervirulence and hypermucoviscosity: Two different but complementary Klebsiella spp. phenotypes?
Catalán-Nájera JC, Garza-Ramos U, Barrios-Camacho H. Catalán-Nájera JC, et al. Virulence. 2017 Oct 3;8(7):1111-1123. doi: 10.1080/21505594.2017.1317412. Epub 2017 Apr 12. Virulence. 2017. PMID: 28402698 Free PMC article. Review.
Cited by
- High Prevalence of Hypervirulent Klebsiella pneumoniae Infection in China: Geographic Distribution, Clinical Characteristics, and Antimicrobial Resistance.
Zhang Y, Zhao C, Wang Q, Wang X, Chen H, Li H, Zhang F, Li S, Wang R, Wang H. Zhang Y, et al. Antimicrob Agents Chemother. 2016 Sep 23;60(10):6115-20. doi: 10.1128/AAC.01127-16. Print 2016 Oct. Antimicrob Agents Chemother. 2016. PMID: 27480857 Free PMC article. - Biofilm Production by Carbapenem-Resistant Klebsiella pneumoniae Significantly Increases the Risk of Death in Oncological Patients.
Di Domenico EG, Cavallo I, Sivori F, Marchesi F, Prignano G, Pimpinelli F, Sperduti I, Pelagalli L, Di Salvo F, Celesti I, Paluzzi S, Pronesti C, Koudriavtseva T, Ascenzioni F, Toma L, De Luca A, Mengarelli A, Ensoli F. Di Domenico EG, et al. Front Cell Infect Microbiol. 2020 Dec 10;10:561741. doi: 10.3389/fcimb.2020.561741. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33363047 Free PMC article. - Complete genome sequence, phenotypic correlation and pangenome analysis of uropathogenic Klebsiella spp.
Sundaresan AK, Gangwar J, Murugavel A, Malli Mohan GB, Ramakrishnan J. Sundaresan AK, et al. AMB Express. 2024 Jul 4;14(1):78. doi: 10.1186/s13568-024-01737-w. AMB Express. 2024. PMID: 38965152 Free PMC article. - Occurrence and Molecular Study of Hypermucoviscous/Hypervirulence Trait in Gut Commensal K. pneumoniae from Healthy Subjects.
Osama DM, Zaki BM, Khalaf WS, Mohamed MYA, Tawfick MM, Amin HM. Osama DM, et al. Microorganisms. 2023 Mar 9;11(3):704. doi: 10.3390/microorganisms11030704. Microorganisms. 2023. PMID: 36985277 Free PMC article. - Multi-antibiotic resistant extended-spectrum beta-lactamase producing bacteria pose a challenge to the effective treatment of wound and skin infections.
Oli AN, Eze DE, Gugu TH, Ezeobi I, Maduagwu UN, Ihekwereme CP. Oli AN, et al. Pan Afr Med J. 2017 May 30;27:66. doi: 10.11604/pamj.2017.27.66.10226. eCollection 2017. Pan Afr Med J. 2017. PMID: 29187917 Free PMC article.
References
- Lee KH, Hui KP, Tan WC, Lim TK. Severe community-acquired pneumonia in Singapore. Singapore Med J. 1996;37(4):374–377. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources