Importazole, a small molecule inhibitor of the transport receptor importin-β - PubMed (original) (raw)
. 2011 Jul 15;6(7):700-8.
doi: 10.1021/cb2000296. Epub 2011 Apr 21.
Affiliations
- PMID: 21469738
- PMCID: PMC3137676
- DOI: 10.1021/cb2000296
Importazole, a small molecule inhibitor of the transport receptor importin-β
Jonathan F Soderholm et al. ACS Chem Biol. 2011.
Abstract
During interphase, the transport receptor importin-β carries cargoes into the nucleus, where RanGTP releases them. A similar mechanism operates in mitosis to generate a gradient of active spindle assembly factors around mitotic chromosomes. Importin-β and RanGTP have been implicated in additional cellular processes, but the precise roles of the Ran/importin-β pathway throughout the cell cycle remain poorly understood. We implemented a FRET-based, high-throughput small molecule screen for compounds that interfere with the interaction between RanGTP and importin-β and identified importazole, a 2,4-diaminoquinazoline. Importazole specifically blocks importin-β-mediated nuclear import both in Xenopus egg extracts and cultured cells, without disrupting transportin-mediated nuclear import or CRM1-mediated nuclear export. When added during mitosis, importazole impairs the release of an importin-β cargo FRET probe and causes both predicted and novel defects in spindle assembly. Together, these results indicate that importazole specifically inhibits the function of importin-β, likely by altering its interaction with RanGTP. Importazole is a valuable tool to evaluate the function of the importin-β/RanGTP pathway at specific stages during the cell cycle.
Figures
Figure 1
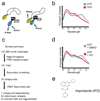
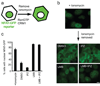
A high-throughput screen identifies importazole as an inhibitor of FRET between CFP-Ran and YFP-importin-β. (a) Schematic of the fusion proteins that bind and undergo FRET in the presence of Ran-GTP but not Ran-GDP. (b) Fluorescence emission of the FRET pair detected between 460 nm and 550 nm following excitation at 435 nm, showing strong emission of CFP (475 nm) in the presence of GDP (red curve) that decreases in the presence of GTP (blue curve) concomitant with an increase at the emission wavelength of YFP (525 nm), indicative of FRET. (c) Summary of the screen. Of 137,284 small molecules screened in duplicate using the FRET-based assay, 141 putative hits were subjected to three secondary screens designed to eliminate false positives. Of the 10 compounds remaining after the secondary screens, only a single compound reproducibly diminished the FRET signal generated by CFP-Ran and YFP-importin-β in the original assay (d). (e) The structure of importazole, a 2,4-diaminoquinazoline.
Figure 2
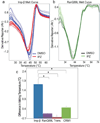
Importazole binds specifically to importin-β. (a) Negative first derivatives of melting curves of 2 µM importin-β in the presence of 50 µM importazole or DMSO where the minima indicate the melting temperature. Melting curves show the results from six experiments conducted in quadruplicate using the Applied Biosystems 7500 qPCR machine. (b) Negative first derivatives of melting curves of 2 µM RanQ69L in the presence of 50 µM importazole or DMSO as control where the minima indicate the melting temperature. (c) Mean changes in melting temperature of 2 µM importin-β, RanQ69L, transportin and CRM1 in the presence of 50 µM importazole. Error bars indicate standard error; asterisks denote statistical significance (p < 0.01).
Figure 3
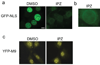
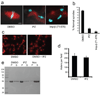
Importazole inhibits importin-β NLS-mediated nuclear import, but not transportin M9-mediated import. (a) NLS-GFP (importin-β import substrate) was added with Xenopus egg extract to permeabilized HeLa cells and assayed by fluorescence microscopy for nuclear import in the presence of DMSO or 100 µm importazole. (b) NLS-GFP accumulation at the nuclear rim in the presence of importazole. (c) M9-YFP (transportin import substrate) in the same assay. In all figures, scale bar = 10 µm.
Figure 4
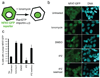
Importazole reversibly blocks importin-β-mediated nuclear import in living cells. (a) Schematic showing that the GFP-tagged, NLS-containing transcription factor NFAT enters the nucleus upon treatment with the ionophore ionomycin in a RanGTP- and importin-β-dependent manner. (b) HEK 293 cells stably expressing GFP-NFAT were treated with DMSO or 40 µm importazole for 1 hour prior to a 30 min treatment with ionomycin to induce nuclear import. Importazole was washed out and after 1 hour prior to ionomycin re-treatment. (c) Results were quantified as the percentage of cells with nuclear NFAT-GFP. N=3, 100 or more cells counted under each condition. Bars represent standard error.
Figure 5
Importazole does not inhibit Crm1-mediated nuclear export. (a) Schematic illustrating that upon removal of the ionophore ionomycin, GFP-NFAT exits the nucleus in a RanGTP- and Crm1-dependent manner. (b) Cells were treated with ionomycin to induce nuclear import of NFAT-GFP, then washed and treated with DMSO, importazole, leptomycin B, or importazole + leptomycin B for 1 hour. (c) Results were quantified as the percentage of cells with nuclear NFAT-GFP. N=3, 100 or more cells counted under each condition. Bars represent standard error.
Figure 6
Importazole impairs spindle assembly in Xenopus egg extracts but does not affect pure microtubule polymerization. (a) Spindle assembly reactions containing X-rhodamine labeled tubulin in the presence of DMSO, 100 µM importazole, or a truncated form of importin-β that is unable to bind to RanGTP. Microtubules are red and DNA is blue. (b) Quantification of the percentage of normal spindle structures. N=3, 100 structures counted under each condition. Bars represent standard error. (c) Aster assembly induced by addition of 5% DMSO to extracts containing X-rhodamine labeled tubulin in the presence of DMSO or importazole. (d) Quantification of the number of asters per field. 10 fields were counted under each condition. (e) DMSO induced pure tubulin polymerization assay. Reactions were supplemented with additional DMSO, importazole, or nocodazole, and microtubules pelleted through a sucrose cushion and samples from the pellet (P) and supernatant (S) analyzed by SDS PAGE.
Figure 7
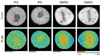
Importazole disrupts mitotic cargo release monitored by the FRET probe Rango. Donor fluorescence (top panels) and pseudo-colored FLIM images (bottom panels) of mitotic HeLa cells expressing the Rango-3 FRET sensor. Rango-3 displays a greater fluorescence lifetime around the chromosomes of cells treated with importazole compared to that of cells treated with DMSO, resulting from importazole’s disruption of sensor release from importin-β. N=3, 30 cells counted for each condition.
Figure 8
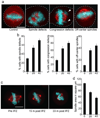
Importazole disrupts mitotic spindles in living HeLa cells. (a) Asynchronously growing cultures were treated with DMSO or importazole for 1 hour prior to fixation and staining for DNA (blue) and tubulin (red). Note defects including chromosome congression (white arrowheads point to misaligned chromosomes) and spindle positioning upon importazole treatment. Dashed white lines indicate cell boundaries. (b) Quantification of spindle defects in cells treated with DMSO, 20 µm importazole, or 40 µm importazole. N=5. In each case, 100 metaphase cells were counted and the fraction of those displaying defects were scored. (c) Time-lapse fluorescence microscopy of a metaphase HeLa cells treated with 50 µm importazole. Frames were captured every 3 minutes. See Supplemental Movie 1. (d) Asynchronous HeLa cells were treated with 0 to 40 µm importazole for 1 hour prior to fixation, and the size of the spindle in mitotic cells was measured. N=4, 100 metaphase spindles were measured per condition. Bars represent standard error.
Similar articles
- The RanGTP gradient - a GPS for the mitotic spindle.
Kalab P, Heald R. Kalab P, et al. J Cell Sci. 2008 May 15;121(Pt 10):1577-86. doi: 10.1242/jcs.005959. J Cell Sci. 2008. PMID: 18469014 Free PMC article. - Transportin acts to regulate mitotic assembly events by target binding rather than Ran sequestration.
Bernis C, Swift-Taylor B, Nord M, Carmona S, Chook YM, Forbes DJ. Bernis C, et al. Mol Biol Cell. 2014 Apr;25(7):992-1009. doi: 10.1091/mbc.E13-08-0506. Epub 2014 Jan 29. Mol Biol Cell. 2014. PMID: 24478460 Free PMC article. - RanGTP and CLASP1 cooperate to position the mitotic spindle.
Bird SL, Heald R, Weis K. Bird SL, et al. Mol Biol Cell. 2013 Aug;24(16):2506-14. doi: 10.1091/mbc.E13-03-0150. Epub 2013 Jun 19. Mol Biol Cell. 2013. PMID: 23783028 Free PMC article. - The role of RanGTP gradient in vertebrate oocyte maturation.
Kaláb P, Solc P, Motlík J. Kaláb P, et al. Results Probl Cell Differ. 2011;53:235-67. doi: 10.1007/978-3-642-19065-0_12. Results Probl Cell Differ. 2011. PMID: 21630149 Review. - Ran-GTP regulates kinetochore attachment in somatic cells.
Arnaoutov A, Dasso M. Arnaoutov A, et al. Cell Cycle. 2005 Sep;4(9):1161-5. doi: 10.4161/cc.4.9.1979. Epub 2005 Sep 28. Cell Cycle. 2005. PMID: 16082212 Review.
Cited by
- Nucleosome functions in spindle assembly and nuclear envelope formation.
Zierhut C, Funabiki H. Zierhut C, et al. Bioessays. 2015 Oct;37(10):1074-85. doi: 10.1002/bies.201500045. Epub 2015 Jul 29. Bioessays. 2015. PMID: 26222742 Free PMC article. Review. - Colocalization of different influenza viral RNA segments in the cytoplasm before viral budding as shown by single-molecule sensitivity FISH analysis.
Chou YY, Heaton NS, Gao Q, Palese P, Singer RH, Lionnet T. Chou YY, et al. PLoS Pathog. 2013;9(5):e1003358. doi: 10.1371/journal.ppat.1003358. Epub 2013 May 9. PLoS Pathog. 2013. PMID: 23671419 Free PMC article. - EIF4G1 and RAN as Possible Drivers for Malignant Pleural Mesothelioma.
Dell'Anno I, Barbarino M, Barone E, Giordano A, Luzzi L, Bottaro M, Migliore L, Agostini S, Melani A, Melaiu O, Catalano C, Cipollini M, Silvestri R, Corrado A, Gemignani F, Landi S. Dell'Anno I, et al. Int J Mol Sci. 2020 Jul 9;21(14):4856. doi: 10.3390/ijms21144856. Int J Mol Sci. 2020. PMID: 32659970 Free PMC article. - SINC, a type III secreted protein of Chlamydia psittaci, targets the inner nuclear membrane of infected cells and uninfected neighbors.
Mojica SA, Hovis KM, Frieman MB, Tran B, Hsia RC, Ravel J, Jenkins-Houk C, Wilson KL, Bavoil PM. Mojica SA, et al. Mol Biol Cell. 2015 May 15;26(10):1918-34. doi: 10.1091/mbc.E14-11-1530. Epub 2015 Mar 18. Mol Biol Cell. 2015. PMID: 25788290 Free PMC article. - A changing paradigm of transcriptional memory propagation through mitosis.
Palozola KC, Lerner J, Zaret KS. Palozola KC, et al. Nat Rev Mol Cell Biol. 2019 Jan;20(1):55-64. doi: 10.1038/s41580-018-0077-z. Nat Rev Mol Cell Biol. 2019. PMID: 30420736 Free PMC article. Review.
References
- Harel A, Forbes DJ. Importin beta: conducting a much larger cellular symphony. Mol Cell. 2004;16:319–330. - PubMed
- Gruss OJ, Carazo-Salas RE, Schatz CA, Guarguaglini G, Kast J, Wilm M, Le Bot N, Vernos I, Karsenti E, Mattaj IW. Ran induces spindle assembly by reversing the inhibitory effect of importin alpha on TPX2 activity. Cell. 2001;104:83–93. - PubMed
- Nachury MV, Maresca TJ, Salmon WC, Waterman-Storer CM, Heald R, Weis K. Importin beta is a mitotic target of the small GTPase Ran in spindle assembly. Cell. 2001;104:95–106. - PubMed
- Wiese C, Wilde A, Moore MS, Adam SA, Merdes A, Zheng Y. Role of importin-beta in coupling Ran to downstream targets in microtubule assembly. Science. 2001;291:653–656. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM065232/GM/NIGMS NIH HHS/United States
- R21 NS053592/NS/NINDS NIH HHS/United States
- R01GM065232/GM/NIGMS NIH HHS/United States
- R21 NS53592/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous