Autophagy in nonalcoholic steatohepatitis - PubMed (original) (raw)
Review
Autophagy in nonalcoholic steatohepatitis
Muhammad Amir et al. Expert Rev Gastroenterol Hepatol. 2011 Apr.
Abstract
Autophagy is a critical pathway for the degradation of intracellular components by lysosomes. Established functions for both macroautophagy and chaperone-mediated autophagy in hepatic lipid metabolism, insulin sensitivity and cellular injury suggest a number of potential mechanistic roles for autophagy in nonalcoholic steatohepatitis (NASH). Decreased autophagic function in particular may promote the initial development of hepatic steatosis and progression of steatosis to liver injury. Additional functions of autophagy in immune responses and carcinogenesis may also contribute to the development of NASH and its complications. The impairment in autophagy that occurs with cellular lipid accumulation, obesity and aging may therefore have an important impact on this disease, and agents to augment hepatic autophagy have therapeutic potential in NASH.
Conflict of interest statement
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Figures
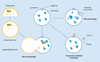
Figure 1. The three types of autophagy
In macroautophagy, a double membrane of unclear origin begins to form a phagophore around cytosolic components, such as mitochondria, lipid droplets and proteins. These cellular elements become completely enclosed within an autophagosome, which translocates to a lysosome full of hydrolases. Fusion of these two structures into an autophagolysosome leads to the enzymatic degradation of the cellular components. In chaperone-mediated autophagy, cytosolic proteins containing a pentapeptide motif bind to the chaperone Hsc70. This complex binds to the LAMP-2A receptor on the lysosome for internalization and degradation. Microautophagy involves the uptake of cellular components within an invagination of the lysosomal membrane for enzymatic degradation. LAMP-2A: Lysosome-associated membrane protein type 2A.
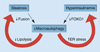
Figure 2. Inter-relationships among cellular changes in lipid content or insulin sensitivity and levels of macroautophagy
In nonalcoholic fatty liver disease, both steatosis and hyperinsulinemia may act to inhibit levels of macroautophagy. Cellular lipid accumulation can inhibit macroautophagy by impeding the fusion of autophagosomes to lysosomes. The mechanism of the inhibitory effect of hyperinsulinemia on autophagy may be through decreased activation of FOXO1. The resultant reduction in macroautophagy may then accelerate steatosis by impairing lipolytic breakdown of lipids stored in lipid droplets. The decrease in macroautophagy may also exacerbate insulin resistance by causing increased ER stress, which worsens hepatic insulin resistance. ER: Endoplasmic reticulum.
Similar articles
- Comprehensive study of the interplay between immunological and metabolic factors in hepatic steatosis.
Banerjee A, Das D, Mukherjee S, Maji BK. Banerjee A, et al. Int Immunopharmacol. 2024 May 30;133:112091. doi: 10.1016/j.intimp.2024.112091. Epub 2024 Apr 23. Int Immunopharmacol. 2024. PMID: 38657500 Review. - Molecular Mechanisms: Connections between Nonalcoholic Fatty Liver Disease, Steatohepatitis and Hepatocellular Carcinoma.
Kanda T, Goto T, Hirotsu Y, Masuzaki R, Moriyama M, Omata M. Kanda T, et al. Int J Mol Sci. 2020 Feb 23;21(4):1525. doi: 10.3390/ijms21041525. Int J Mol Sci. 2020. PMID: 32102237 Free PMC article. Review. - Immunological and molecular basis of nonalcoholic steatohepatitis and nonalcoholic fatty liver disease.
Radwan MM, Radwan BM, Nandipati KC, Hunter WJ 3rd, Agrawal DK. Radwan MM, et al. Expert Rev Clin Immunol. 2013 Aug;9(8):727-38. doi: 10.1586/1744666X.2013.816484. Expert Rev Clin Immunol. 2013. PMID: 23971751 Review. - Gemcabene downregulates inflammatory, lipid-altering and cell-signaling genes in the STAM™ model of NASH.
Oniciu DC, Hashiguchi T, Shibazaki Y, Bisgaier CL. Oniciu DC, et al. PLoS One. 2018 May 30;13(5):e0194568. doi: 10.1371/journal.pone.0194568. eCollection 2018. PLoS One. 2018. PMID: 29847555 Free PMC article. - The ménage à trois of autophagy, lipid droplets and liver disease.
Filali-Mouncef Y, Hunter C, Roccio F, Zagkou S, Dupont N, Primard C, Proikas-Cezanne T, Reggiori F. Filali-Mouncef Y, et al. Autophagy. 2022 Jan;18(1):50-72. doi: 10.1080/15548627.2021.1895658. Epub 2021 Apr 2. Autophagy. 2022. PMID: 33794741 Free PMC article. Review.
Cited by
- Efficacy of MK615 for the treatment of patients with liver disorders.
Hokari A, Ishikawa T, Tajiri H, Matsuda T, Ishii O, Matsumoto N, Okuse C, Takahashi H, Kurihara T, Kawahara K, Maruyama I, Zeniya M. Hokari A, et al. World J Gastroenterol. 2012 Aug 21;18(31):4118-26. doi: 10.3748/wjg.v18.i31.4118. World J Gastroenterol. 2012. PMID: 22919243 Free PMC article. Clinical Trial. - Modified Genomic Self-DNA Influences In Vitro Survival of HT29 Tumor Cells via TLR9- and Autophagy Signaling.
Sipos F, Kiss AL, Constantinovits M, Tulassay Z, Műzes G. Sipos F, et al. Pathol Oncol Res. 2019 Oct;25(4):1505-1517. doi: 10.1007/s12253-018-0544-z. Epub 2018 Nov 21. Pathol Oncol Res. 2019. PMID: 30465163 - Mitochondria and Reactive Oxygen Species in Aging and Age-Related Diseases.
Giorgi C, Marchi S, Simoes ICM, Ren Z, Morciano G, Perrone M, Patalas-Krawczyk P, Borchard S, Jędrak P, Pierzynowska K, Szymański J, Wang DQ, Portincasa P, Węgrzyn G, Zischka H, Dobrzyn P, Bonora M, Duszynski J, Rimessi A, Karkucinska-Wieckowska A, Dobrzyn A, Szabadkai G, Zavan B, Oliveira PJ, Sardao VA, Pinton P, Wieckowski MR. Giorgi C, et al. Int Rev Cell Mol Biol. 2018;340:209-344. doi: 10.1016/bs.ircmb.2018.05.006. Epub 2018 Jun 22. Int Rev Cell Mol Biol. 2018. PMID: 30072092 Free PMC article. Review. - Inflammatory and fibrotic mechanisms in NAFLD-Implications for new treatment strategies.
Lee YA, Friedman SL. Lee YA, et al. J Intern Med. 2022 Jan;291(1):11-31. doi: 10.1111/joim.13380. Epub 2021 Sep 26. J Intern Med. 2022. PMID: 34564899 Free PMC article. Review.
References
- Mehrpour M, Esclatine A, Beau I, Codogno P. Autophagy in health and disease. 1. Regulation and significance of autophagy: an overview. Am. J. Physiol. Cell Physiol. 2010;298(4):C776–C785. - PubMed
- Komatsu M, Waguri S, Chiba T, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441(7095):880–884. - PubMed
- Finn PF, Dice JF. Proteolytic and lipolytic responses to starvation. Nutrition. 2006;22(7–8):830–844. • Excellent review of the function of autophagy during times of nutrient deprivation.
- Iwata J, Ezaki J, Komatsu M, et al. Excess peroxisomes are degraded by autophagic machinery in mammals. J. Biol. Chem. 2006;281(7):4035–4041. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources