Evidence for presence and functional effects of Kv1.1 channels in β-cells: general survey and results from mceph/mceph mice - PubMed (original) (raw)
Evidence for presence and functional effects of Kv1.1 channels in β-cells: general survey and results from mceph/mceph mice
Zuheng Ma et al. PLoS One. 2011.
Abstract
Background: Voltage-dependent K(+) channels (Kv) mediate repolarisation of β-cell action potentials, and thereby abrogate insulin secretion. The role of the Kv1.1 K(+) channel in this process is however unclear. We tested for presence of Kv1.1 in different species and tested for a functional role of Kv1.1 by assessing pancreatic islet function in BALB/cByJ (wild-type) and megencephaly (mceph/mceph) mice, the latter having a deletion in the Kv1.1 gene.
Methodology/principal findings: Kv1.1 expression was detected in islets from wild-type mice, SD rats and humans, and expression of truncated Kv1.1 was detected in mceph/mceph islets. Full-length Kv1.1 protein was present in islets from wild-type mice, but, as expected, not in those from mceph/mceph mice. Kv1.1 expression was localized to the β-cell population and also to α- and δ-cells, with evidence of over-expression of truncated Kv1.1 in mceph/mceph islets. Blood glucose, insulin content, and islet morphology were normal in mceph/mceph mice, but glucose-induced insulin release from batch-incubated islets was (moderately) higher than that from wild-type islets. Reciprocal blocking of Kv1.1 by dendrotoxin-K increased insulin secretion from wild-type but not mceph/mceph islets. Glucose-induced action potential duration, as well as firing frequency, was increased in mceph/mceph mouse β-cells. This duration effect on action potential in β-cells from mceph/mceph mice was mimicked by dendrotoxin-K in β-cells from wild-type mice. Observations concerning the effects of both the mceph mutation, and of dendrotoxin-K, on glucose-induced insulin release were confirmed in pancreatic islets from Kv1.1 null mice.
Conclusion/significance: Kv1.1 channels are expressed in the β-cells of several species, and these channels can influence glucose-stimulated insulin release.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
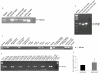
Figure 1. Kv1.1 is expressed in mceph/mceph mice.
Kv1.1 mRNA expression in islets from wild-type and mceph/mceph mice by RT-PCR with the S2645-AS3455 (A) and S2688-AS2912 (B) primer pairs, which amplify sequences not including, and including, the mceph mutation respectively. Marker = pUC18 Msp I digest. Kv1.1 is expressed in mice, rat and human islets as well as INS-1 cells but not MIN6 cells. Kv1.1 mRNA expression by RT-PCR with the S2645-AS3455 primer pair (C). Marker = pUC18 Msp I digest. (D) Kv1.1 mRNA expression in islets isolated from three human control and three diabetic individuals by RT-PCR with the S1926-AS2092 primer pair. Marker = pUC18 MspI digest. (E) Kv1.1 mRNA expression levels relative to 18S RNA by real-time PCR in islets from human non-diabetic (n = 3) and diabetic individuals (n = 3). Samples were run in duplicates. All analyses included a corresponding negative control (RT-ve).
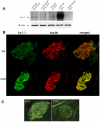
Figure 2. Kv1.1 protein is present in islets from wild type mice and rats.
Western blotting using a monoclonal antibody against the C-terminal of Kv1.1 protein (A). Brain from wild type and mceph/mceph mice was used as controls. mceph/mceph served as specificity control as these mice express only the N-terminal of Kv1.1. β-actin was used as loading control. Kv1.1 protein is present in β-cells. Islets from wild type mice (wt) (B upper panel) and mceph/mceph (m/m) mice (B lower panel) double immunostained for N-terminal Kv1.1 (green, left) and insulin (red, middle); merged to the right. The N-terminal of Kv1.1 is present in beta cells of both genotypes. Note that Kv1.1 is up-regulated in mceph/mceph mice compared to wild type mice. Specificity of the N-terminal Kv1.1-LI was tested by determining the level of Kv1.2-LI. Strong N-terminal Kv1.1-LI and very weak Kv1.2-LI in an islet from mceph/mceph mice assured that the Kv1.1-LI reflected presence of Kv1.1 (C).
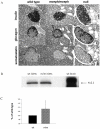
Figure 3. Normal islet structure in mceph/mceph and Kv 1.1 null mice compared to wild type mice.
Medium-power photomicrographs, showing- all at the same magnification x400-serial sections of an islet of Langerhans in the pancreatic parenchyma from mice belonging to the three strains (A). The fact that the islet chosen for this particular set of photomicrographs happens to be larger from the Kv1.1 null mouse strain than those from the other two strains, is by pure incidence only, and does not, by no means, imply that the Kv1.1 null islets in general would be larger than those in a normal murine pancreas. Over-expression of truncated Kv1.1 protein in mceph/mceph does not affect the expression of Kv2.1 protein. Western blot with a polyclonal antibody against Kv2.1 protein in islets from mceph/mceph. Column diagram shows compiled data from 3 individual experiments (C), blots are from a typical experiment (B).
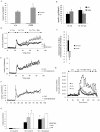
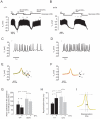
Figure 4. Increased glucose-induced insulin secretion in islets from mceph/mceph vs. wild type mice.
Glucose-induced insulin secretion from
batch-type incubations
of islets of Langerhans from wild-type (+/+) and mceph/mceph (m/m) mice, n = 11 (A). *P<0.03 vs. wild type. Dendrotoxin-K increases insulin secretion in wild type but not in mceph/mceph and Kv1.1 null mouse islets. Incremental insulin release from batch-type incubations of islets from wild-type (wt) and mceph/mceph (m/m) mice in response to 10 nmol/l dendrotoxin-K (dtx), n = 7 (B). The difference in response to dendrotoxin-K for wild-type and mceph/mceph mice was significant, *P = 0.02 (C). Each batch-type experiment was performed in triplicate or quadruplicate with three equal-sized islets per tube. Equal amounts of islets from wild-type (D, n = 4), mutant mceph/mceph (E, n = 4) or Kv1.1 null (F, n = 3) mice were loaded into separate
perifusion
chambers. For typographical reasons only every fifth error bar is shown in (E and F). The response to 10 nmol/l dendrotoxin-K in wild-type islets was significant (*P = 0.05). For details of how the dendrotoxin-K-effect was calculated see results. (G) summarises average insulin response to glucose level elevation from 3.3 to 16.7 mmol/l as well as addition of dendrotoxin-K in the islet perifusion experiments. (The insulin response to an increase in glucose concentration from 3.3 to 16.7 mmol/l was significantly increased in islets from mceph/mceph and Kv1.1 null vs. wild-type mice (*P = 0.01). The response to dendrotoxin-K in wild-type islets was significant (†P = 0.05), while that was not the case in mceph/mceph and Kv1.1 null islets. Increased insulin response to dendrotoxin-K and TEA in dispersed islet cells from wild type mice. Perifusion with 200 nmol/l dendrotoxin-K or 15 mmol/l TEA. 0.5 mmol/l IBMX was present from min 5 to 51. One typical experiment out of three is shown (H).
Figure 5. Dendrotoxin-K significantly blocks relative K+ currents in wild type vs. mceph/mceph β-cells.
Whole-cell patch-clamp recordings from wild-type and mceph/mceph pancreatic β-cells. I-V relationship for wild-type (A) and mceph/mceph (B) pancreatic β-cells before, during and after exposure to 20 nmol/l dendrotoxin-K were obtained by clamping the cells at −80 mV and subsequently depolarized in steps of 20 mV for 100 ms to 80 mV. Example traces are recorded from wild-type β-cell in (C) at +80 mV, and the current measured at +80 mV is plotted every 20 s (D). In (E), to compensate for channel run-down, in each recording whole-cell K+ current was normalized to the estimated K+ current by extrapolating the current before and after the toxin administration.*P<0.05; n.s., not significant.
Figure 6. mceph/mceph β-cells display a higher action potential frequency and a prolongation of repolarization vs. wild-type β-cells.
Membrane potential were recorded in pancreatic β-cells isolated from wild-type mouse (A) and mceph/mceph mouse (B). (C) and (D), displays recordings of membrane potential on an expanded time scale during 15 mmol/l glucose (i). (E) and (F) show summary of 20 action potentials at 15 mmol/glucose +/−20 nmol/l dendrotoxin-K (indicated by roman numbers i, ii, iii and iv). (G), action potential frequency (per 60 s) in 15 mmol/l glucose in β-cells isolated from wild-type (n = 4) and mceph/mceph (n = 6), with and without 20 nmol/l dendrotoxin-K (n = 3, both groups). (H) shows compiled data of repolarisation phase in wild-type and mceph/mceph β-cells, measured as time lag from action potential peak and 2/3 of action potential amplitude (see schematic drawing in (I)). All analysis of action potential characteristics were performed using sections of membrane potential recording in close proximity and prior to change of solution. *P<0.05; n.s., not significant.
Similar articles
- A truncated Kv1.1 protein in the brain of the megencephaly mouse: expression and interaction.
Persson AS, Klement G, Almgren M, Sahlholm K, Nilsson J, Petersson S, Arhem P, Schalling M, Lavebratt C. Persson AS, et al. BMC Neurosci. 2005 Nov 23;6:65. doi: 10.1186/1471-2202-6-65. BMC Neurosci. 2005. PMID: 16305740 Free PMC article. - Truncation of the Shaker-like voltage-gated potassium channel, Kv1.1, causes megencephaly.
Petersson S, Persson AS, Johansen JE, Ingvar M, Nilsson J, Klement G, Arhem P, Schalling M, Lavebratt C. Petersson S, et al. Eur J Neurosci. 2003 Dec;18(12):3231-40. doi: 10.1111/j.1460-9568.2003.03044.x. Eur J Neurosci. 2003. PMID: 14686897 - Members of the Kv1 and Kv2 voltage-dependent K(+) channel families regulate insulin secretion.
MacDonald PE, Ha XF, Wang J, Smukler SR, Sun AM, Gaisano HY, Salapatek AM, Backx PH, Wheeler MB. MacDonald PE, et al. Mol Endocrinol. 2001 Aug;15(8):1423-35. doi: 10.1210/mend.15.8.0685. Mol Endocrinol. 2001. PMID: 11463864 - Beta-cell ion channels: keys to endodermal excitability.
Philipson LH. Philipson LH. Horm Metab Res. 1999 Aug;31(8):455-61. doi: 10.1055/s-2007-978774. Horm Metab Res. 1999. PMID: 10494870 Review. - Voltage-dependent K(+) channels in pancreatic beta cells: role, regulation and potential as therapeutic targets.
MacDonald PE, Wheeler MB. MacDonald PE, et al. Diabetologia. 2003 Aug;46(8):1046-62. doi: 10.1007/s00125-003-1159-8. Epub 2003 Jun 27. Diabetologia. 2003. PMID: 12830383 Review.
Cited by
- First genome-wide association study in an Australian aboriginal population provides insights into genetic risk factors for body mass index and type 2 diabetes.
Anderson D, Cordell HJ, Fakiola M, Francis RW, Syn G, Scaman ES, Davis E, Miles SJ, McLeay T, Jamieson SE, Blackwell JM. Anderson D, et al. PLoS One. 2015 Mar 11;10(3):e0119333. doi: 10.1371/journal.pone.0119333. eCollection 2015. PLoS One. 2015. PMID: 25760438 Free PMC article. - Kv1.1 Channelopathies: Pathophysiological Mechanisms and Therapeutic Approaches.
D'Adamo MC, Liantonio A, Rolland JF, Pessia M, Imbrici P. D'Adamo MC, et al. Int J Mol Sci. 2020 Apr 22;21(8):2935. doi: 10.3390/ijms21082935. Int J Mol Sci. 2020. PMID: 32331416 Free PMC article. Review. - Interactions of the Kv1.1 Channel with Peptide Pore Blockers: A Fluorescent Analysis on Mammalian Cells.
Orlov NA, Kryukova EV, Efremenko AV, Yakimov SA, Toporova VA, Kirpichnikov MP, Nekrasova OV, Feofanov AV. Orlov NA, et al. Membranes (Basel). 2023 Jul 4;13(7):645. doi: 10.3390/membranes13070645. Membranes (Basel). 2023. PMID: 37505011 Free PMC article.
References
- MacDonald PE, Ha PX, Wang J, Smukler SR, Sun AM, et al. Members of the Kv1 and Kv2 Voltage-Dependent K+ Channel Families Regulate Insulin Secretion. Mol Endocrinol. 2001;15:1423–1435. - PubMed
- Escobar LI, Martı'nez-Te'llez JC, Salas M, Castilla SA, Carrisoza R, et al. A voltage-gated K(+) current in renal inner medullary collecting duct cells. Am J Physiol Cell Physiol. 2004;286:C965–74. - PubMed
- MacDonald PE, Wheeler MB. Voltage-dependent K channels in pancreatic beta cells: Role, regulation and potential as therapeutic targets. Diabetologia. 2003;46:1046–1062. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases