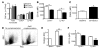Suppressed monocyte recruitment drives macrophage removal from atherosclerotic plaques of Apoe-/- mice during disease regression - PubMed (original) (raw)
. 2011 May;121(5):2025-36.
doi: 10.1172/JCI43802. Epub 2011 Apr 18.
Affiliations
- PMID: 21505265
- PMCID: PMC3083793
- DOI: 10.1172/JCI43802
Suppressed monocyte recruitment drives macrophage removal from atherosclerotic plaques of Apoe-/- mice during disease regression
Stephane Potteaux et al. J Clin Invest. 2011 May.
Abstract
Experimental models of atherosclerosis suggest that recruitment of monocytes into plaques drives the progression of this chronic inflammatory condition. Cholesterol-lowering therapy leads to plaque stabilization or regression in human atherosclerosis, characterized by reduced macrophage content, but the mechanisms that underlie this reduction are incompletely understood. Mice lacking the gene Apoe (Apoe-/- mice) have high levels of cholesterol and spontaneously develop atherosclerotic lesions. Here, we treated Apoe-/- mice with apoE-encoding adenoviral vectors that induce plaque regression, and investigated whether macrophage removal from plaques during this regression resulted from quantitative alterations in the ability of monocytes to either enter or exit plaques. Within 2 days after apoE complementation, plasma cholesterol was normalized to wild-type levels, and HDL levels were increased 4-fold. Oil red O staining and quantitative mass spectroscopy revealed that esterified cholesterol content was markedly reduced. Plaque macrophage content decreased gradually and was 72% lower than baseline 4 weeks after apoE complementation. Importantly, this reduction in macrophages did not involve migratory egress from plaques or CCR7, a mediator of leukocyte emigration. Instead, marked suppression of monocyte recruitment coupled with a stable rate of apoptosis accounted for loss of plaque macrophages. These data suggest that therapies to inhibit monocyte recruitment to plaques may constitute a more viable strategy to reduce plaque macrophage burden than attempts to promote migratory egress.
Figures
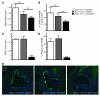
Figure 1. Kinetics of changes within Apoe–/– plaques following apoE complementation.
Apoe–/– mice were fed a HFD for 9 weeks before establishment of a baseline group or starting treatment with ad-hApoE3 or ad-Empty vector. The evolution of changes within plaque after 1, 2, or 4 weeks following adenoviral infection was characterized. (A) Total cholesterol measurements. Dashed line depicts the values obtained from age- and sex-matched C57BL/6 Apoe+/+ mice. (B) The plasma lipoprotein profile as a function of time following apoE complementation. (C) Lesion area was measured on 8-μm sections at 48-μm intervals, starting from the initiation of valves. (D) Macrophage area was quantified by CD68+ area and normalized to the average CD68+ area from the corresponding baseline group lesions. (E) Representative photomicrographs of CD68+ staining in sections of the aortic sinus of each experimental group. Red, CD68; blue, DAPI. Original magnification, ×50. (F) Neutral lipid area within lesions was quantified by ORO staining within each treatment group over time; the dashed line refers to baseline values obtained in Apoe–/– mice after 9 weeks of HFD feeding. (G and H) Total, free, and esterified cholesterol mass analysis at baseline or 14 days after apoE complementation normalized to wet weight of tissue (G) or tissue protein content (H) from 7–8 mice per group. Data represent mean ± SEM, except in A, which depicts mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001. Results are compiled from 3 independent experiments with 5–8 animals per group per experiment.
Figure 2. CCR7-independent removal of Apoe–/– plaque macrophages after apoE complementation.
Apoe–/–Ccr7–/– and Apoe–/–Ccr7+/+ (littermate control) mice were fed HFD for 9 weeks. A cohort of animals from each genotype was sacrificed to establish baseline (white and gray bars). A second cohort of Apoe–/–Ccr7–/– was then infected with ad-hApoE3 vector for 4 weeks (black bar) before sacrifice. (A) Plaque area was quantified in aortic sinus sections and (B) en face aortic arch preparation. (C) Lipid area was measured by ORO coloration and (D) macrophage content by CD68+staining. (E) Representative photomicrographs of macrophage (CD68+) area in sections of the aortic sinus at time of sacrifice in each group. Green, CD68; blue, DAPI. Original magnification, ×50. Data represent mean ± SEM from 2 independent experiments; n = 3 mice in Apoe–/–Ccr7+/+ baseline group, n = 5 in Apoe–/–Ccr7–/– baseline group, n = 6 in Apoe–/–Ccr7–/– ad-hApoE3 group; ***P < 0.001.
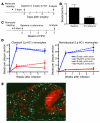
Figure 3. Analysis of migratory egress from atherosclerotic plaques in Apoe–/– mice following apoE complementation.
(A) Diagram depicts experimental design in which the Ly-6Clo monocytes of mice were labeled with beads in the blood before the mice served as transplant donors in a surgical model of regression. (B) Quantification of the number of beads per cross section of the aortic arch before (baseline) and after plaque regression in WT transplant recipients. Data were pooled from 3 independent experiments; n = 5 in the baseline group, n = 7 in the WT recipient group. Difference from baseline is significant, P < 0.01. (C) Experimental design to study migratory egress from plaques of Apoe–/– mice after apoE complementation. (D) Quantification of bead number in aortic sinus plaques (blue lines) or aortic arch (lesser curvature; red lines) after Ly-6Chi (upper graph; n = 5–7 mice per data point) or Ly-6Clo (lower graph; n = 10–16 mice per data point) monocytes were initially labeled in the blood. (E) Microphotograph of beads (green particles, some indicated by arrows) localized within an aortic arch plaque 4 weeks after labeling in the control group. Non-overlapping ORO (red) and DAPI (blue) staining reveals necrotic core. Data represent mean ± SEM.
Figure 4. Analysis of blood monocyte numbers and phenotype following apoE complementation.
(A) Total monocytes and monocyte subsets were quantified by flow cytometry in blood of baseline mice or 4 weeks after infection with ad-Empty and ad-hApoE3 (n = 10–34 mice per bar). (B) CD11b and (C) CD62L (Ly-6Chi monocytes only) expression was analyzed with (black bars) or without apoE complementation (white bars) of Apoe–/– mice maintained on a HFD. n = 5–9 mice per bar; *P < 0.01. (D) Flow plot overlays show whole leukocyte fraction in blood (gray) overlaid with profiles of Ly-6Clo monocytes (black) from Apoe–/– mice fed a HFD followed by apoE complementation or no complementation. (E) Summary of relative changes in SSC-A shifts in Ly-6Clo monocytes from Apoe–/– mice on a HFD complemented (black bar) or not (white bars) with apoE-encoding vector. Data are compared with SSC-A of monocytes from Apoe+/+ control mice. (F) Effect of hApoE3 complementation on surface expression of CD115. n = 5 mice per bar; **P < 0.001.
Figure 5. Monocyte recruitment and apoptotic death during macrophage loss from plaques.
(A) Effect of ad-Empty vector infection on Ly-6Clo monocyte recruitment into the plaque using the bead labeling assay. n = 5–7 mice/group. (B) Recruitment of Ly-6Clo monocytes was quantified 1, 2, and 4 weeks (10, 11, and 13 weeks of HFD) after ad-hApoE3 infection and ad-Empty infection. Monocytes were labeled 2–6 days before sacrifice, depending on the experiment. Relative bead number was obtained by dividing the number of beads for each mouse by the number of beads averaged from the corresponding control ad-Empty group. Data represent 4 experiments; n = 5–7 mice per group. (C) Recruitment of Ly-6Chi monocytes was quantified 2 weeks after ad-hApoE3 infection and ad-Empty infection. n = 5–7 mice/group. (D) Quantification of the number of CD68+ TUNEL+ DAPI+ cells per section in the aortic sinus, 2 weeks after ad-Empty or ad-hApoE3 infection (n = 3–7/group). (E) Lesion area of chow-fed Apoe–/– mice, with baseline group analysis conducted at 31 weeks of age and further analysis 2 or 4 weeks after ad-hApoE3 vector infection; n = 5 mice per group. (F) Total plasma cholesterol in 17-week-old Apoe–/– mice fed a HFD, 31-week-old Apoe–/– mice fed a chow diet, and WT mice fed a chow diet (white bars). Additional measurements were made 2 weeks after ah-hApoE3 infection (black bars); n = 5 per group. (G) Fraction of lesion area, from Apoe–/– animals in F, containing CD68+ cells. (H) Relative effect of ad-hApoE3 in altering monocyte recruitment in chow-fed Apoe–/– mice, using same approach as in B for HFD-fed Apoe–/– mice. (I) Correlation between total plasma cholesterol and monocyte recruitment measured after bead labeling of blood monocytes. Correlation is significant; P < 0.001. Data in all panels represent mean ± SEM; *P < 0.01; **P < 0.001.
Figure 6. Effect of apoE complementation on the expression of adhesion molecules in atherosclerotic plaque.
Immunofluorescent staining of ICAM-1, VCAM-1, and osteopontin (red) in aortic sinus lesions was carried out and quantified. Blue, nuclei. Original magnification, ×100 for ICAM-1 and VCAM-1, and ×50 for osteopontin. Quantifications of red area in a series of sections in 5 animals per stain are shown below each representative micrograph. All reductions in staining intensity of adhesion molecules were statistically significant, with a P value of 0.01 or less.
Similar articles
- Deletion of Macrophage Low-Density Lipoprotein Receptor-Related Protein 1 (LRP1) Accelerates Atherosclerosis Regression and Increases C-C Chemokine Receptor Type 7 (CCR7) Expression in Plaque Macrophages.
Mueller PA, Zhu L, Tavori H, Huynh K, Giunzioni I, Stafford JM, Linton MF, Fazio S. Mueller PA, et al. Circulation. 2018 Oct 23;138(17):1850-1863. doi: 10.1161/CIRCULATIONAHA.117.031702. Circulation. 2018. PMID: 29794082 Free PMC article. - Shear stress-induced atherosclerotic plaque composition in ApoE(-/-) mice is modulated by connexin37.
Pfenniger A, Meens MJ, Pedrigi RM, Foglia B, Sutter E, Pelli G, Rochemont V, Petrova TV, Krams R, Kwak BR. Pfenniger A, et al. Atherosclerosis. 2015 Nov;243(1):1-10. doi: 10.1016/j.atherosclerosis.2015.08.029. Epub 2015 Aug 25. Atherosclerosis. 2015. PMID: 26342936 - HDL promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocyte-derived cells.
Feig JE, Rong JX, Shamir R, Sanson M, Vengrenyuk Y, Liu J, Rayner K, Moore K, Garabedian M, Fisher EA. Feig JE, et al. Proc Natl Acad Sci U S A. 2011 Apr 26;108(17):7166-71. doi: 10.1073/pnas.1016086108. Epub 2011 Apr 11. Proc Natl Acad Sci U S A. 2011. PMID: 21482781 Free PMC article. - Macrophages in atherosclerosis: a dynamic balance.
Moore KJ, Sheedy FJ, Fisher EA. Moore KJ, et al. Nat Rev Immunol. 2013 Oct;13(10):709-21. doi: 10.1038/nri3520. Epub 2013 Sep 2. Nat Rev Immunol. 2013. PMID: 23995626 Free PMC article. Review. - Therapeutic strategies to deplete macrophages in atherosclerotic plaques.
De Meyer I, Martinet W, De Meyer GR. De Meyer I, et al. Br J Clin Pharmacol. 2012 Aug;74(2):246-63. doi: 10.1111/j.1365-2125.2012.04211.x. Br J Clin Pharmacol. 2012. PMID: 22309283 Free PMC article. Review.
Cited by
- Endoplasmic reticulum stress controls M2 macrophage differentiation and foam cell formation.
Oh J, Riek AE, Weng S, Petty M, Kim D, Colonna M, Cella M, Bernal-Mizrachi C. Oh J, et al. J Biol Chem. 2012 Apr 6;287(15):11629-41. doi: 10.1074/jbc.M111.338673. Epub 2012 Feb 22. J Biol Chem. 2012. PMID: 22356914 Free PMC article. - Effects of High Fat Feeding and Diabetes on Regression of Atherosclerosis Induced by Low-Density Lipoprotein Receptor Gene Therapy in LDL Receptor-Deficient Mice.
Willecke F, Yuan C, Oka K, Chan L, Hu Y, Barnhart S, Bornfeldt KE, Goldberg IJ, Fisher EA. Willecke F, et al. PLoS One. 2015 Jun 5;10(6):e0128996. doi: 10.1371/journal.pone.0128996. eCollection 2015. PLoS One. 2015. PMID: 26046657 Free PMC article. - Quantitative analysis of monocyte subpopulations in murine atherosclerotic plaques by multiphoton microscopy.
Haka AS, Potteaux S, Fraser H, Randolph GJ, Maxfield FR. Haka AS, et al. PLoS One. 2012;7(9):e44823. doi: 10.1371/journal.pone.0044823. Epub 2012 Sep 14. PLoS One. 2012. PMID: 23024767 Free PMC article. - HDL and Reverse Cholesterol Transport.
Ouimet M, Barrett TJ, Fisher EA. Ouimet M, et al. Circ Res. 2019 May 10;124(10):1505-1518. doi: 10.1161/CIRCRESAHA.119.312617. Circ Res. 2019. PMID: 31071007 Free PMC article. Review. - Hematopoiesis and Cardiovascular Disease.
Poller WC, Nahrendorf M, Swirski FK. Poller WC, et al. Circ Res. 2020 Apr 10;126(8):1061-1085. doi: 10.1161/CIRCRESAHA.120.315895. Epub 2020 Apr 9. Circ Res. 2020. PMID: 32271679 Free PMC article. Review.
References
- Farb A, et al. Coronary plaque erosion without rupture into a lipid core. A frequent cause of coronary thrombosis in sudden coronary death. Circulation. 1996;93(7):1354–1363. - PubMed
- Shah PK. Pathophysiology of plaque rupture and the concept of plaque stabilization. Cardiol Clin. 1996;14(1):17–29. - PubMed
- Libby P, Schoenbeck U, Mach F, Selwyn AP, Ganz P. Current concepts in cardiovascular pathology: the role of LDL cholesterol in plaque rupture and stabilization. Am J Med. 1998;104(2A):14S–18S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL096539/HL/NHLBI NIH HHS/United States
- P01 HL049373/HL/NHLBI NIH HHS/United States
- HL-64163/HL/NHLBI NIH HHS/United States
- AI049653/AI/NIAID NIH HHS/United States
- HL-49373/HL/NHLBI NIH HHS/United States
- R01 AI061741/AI/NIAID NIH HHS/United States
- AI061741/AI/NIAID NIH HHS/United States
- R01 HL064163/HL/NHLBI NIH HHS/United States
- R01 AI049653/AI/NIAID NIH HHS/United States
- R01 HL112276/HL/NHLBI NIH HHS/United States
- HL096539/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous