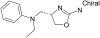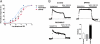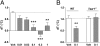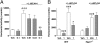TAAR1 activation modulates monoaminergic neurotransmission, preventing hyperdopaminergic and hypoglutamatergic activity - PubMed (original) (raw)
. 2011 May 17;108(20):8485-90.
doi: 10.1073/pnas.1103029108. Epub 2011 Apr 27.
Jean-Luc Moreau, Raul R Gainetdinov, Amyaouch Bradaia, Tatyana D Sotnikova, Roland Mory, Sean Durkin, Katrin Groebke Zbinden, Roger Norcross, Claas A Meyer, Veit Metzler, Sylvie Chaboz, Laurence Ozmen, Gerhard Trube, Bruno Pouzet, Bernhard Bettler, Marc G Caron, Joseph G Wettstein, Marius C Hoener
Affiliations
- PMID: 21525407
- PMCID: PMC3101002
- DOI: 10.1073/pnas.1103029108
TAAR1 activation modulates monoaminergic neurotransmission, preventing hyperdopaminergic and hypoglutamatergic activity
Florent G Revel et al. Proc Natl Acad Sci U S A. 2011.
Abstract
The trace amine-associated receptor 1 (TAAR1), activated by endogenous metabolites of amino acids like the trace amines p-tyramine and β-phenylethylamine, has proven to be an important modulator of the dopaminergic system and is considered a promising target for the treatment of neuropsychiatric disorders. To decipher the brain functions of TAAR1, a selective TAAR1 agonist, RO5166017, was engineered. RO5166017 showed high affinity and potent functional activity at mouse, rat, cynomolgus monkey, and human TAAR1 stably expressed in HEK293 cells as well as high selectivity vs. other targets. In mouse brain slices, RO5166017 inhibited the firing frequency of dopaminergic and serotonergic neurons in regions where Taar1 is expressed (i.e., the ventral tegmental area and dorsal raphe nucleus, respectively). In contrast, RO5166017 did not change the firing frequency of noradrenergic neurons in the locus coeruleus, an area devoid of Taar1 expression. Furthermore, modulation of TAAR1 activity altered the desensitization rate and agonist potency at 5-HT(1A) receptors in the dorsal raphe, suggesting that TAAR1 modulates not only dopaminergic but also serotonergic neurotransmission. In WT but not Taar1(-/-) mice, RO5166017 prevented stress-induced hyperthermia and blocked dopamine-dependent hyperlocomotion in cocaine-treated and dopamine transporter knockout mice as well as hyperactivity induced by an NMDA antagonist. These results tie TAAR1 to the control of monoamine-driven behaviors and suggest anxiolytic- and antipsychotic-like properties for agonists such as RO5166017, opening treatment opportunities for psychiatric disorders.
Conflict of interest statement
Conflict of interest statement: F.G.R., J.-L.M., R.M., S.D., K.G.Z., R.N., C.A.M., V.M., S.C., L.O., G.T., B.P., J.G.W., and M.C.H. are F. Hoffmann-La Roche employees. R.R.G. is supported in part by research grants from F. Hoffmann-La Roche Ltd. (Basel, Switzerland) and Compagnia di San Paolo Fondazione (Turin, Italy). A.B. is employed by Neuroservice. M.G.C. has received funds for Sponsored Research Agreements unrelated to this work from Forest Laboratories, NeuroSearch, and Lundbeck USA as well as consulting fees from Merck and F. Hoffmann-La Roche Ltd. An unrestricted gift to Duke University was provided by Lundbeck USA to support Neuroscience research in the laboratory of M.G.C.
Figures
Fig. 1.
Chemical structure of the selective TAAR1 agonist RO5166017.
Fig. 2.
RO5166017 inhibits the firing rate of DA and 5-HT neurons but not that of NA neurons. (A, C, and E) Representative recordings from brain slices of WT and Taar1−/− mice. (Scale bar: VTA, 20 mV/s; DRN and LC, 30 mV/s.) (B, D, and F) Quantification bar graphs (mean ± SEM; n = 5 neurons, recorded from three animals per condition). The firing frequency was assessed before (Control) and during application of RO5166017 (500 nM; RO) alone and in combination with EPPTB (10 nM). In WT mice, RO5166017 decreased firing of DA neurons in the VTA (A and B) and 5-HT neurons in the DRN (C and D), whereas application of EPPTB increased the firing rate above control levels. In brain slices of Taar1−/− mice, the spontaneous firing frequencies of the DA and 5-HT neurons were increased compared with WT, and they were not affected by RO5166017. In the LC (E and F), the spontaneous firing rates of NA neurons in WT and Taar1−/− mice were not significantly different, and RO5166017 had no effect in WT mice. ***P < 0.001 vs. the other two conditions.
Fig. 3.
TAAR1 activity modulates the 5-HT1A receptor pharmacology in 5-HT neurons. (A) Dose–response relationships of the currents induced by the 5-HT1A receptor agonist ipsapirone recorded from DRN 5-HT neurons in control slices and slices preincubated with EPPTB (10 nM) or RO5166017 (19 nM). Current amplitudes were normalized to the maximal current obtained with a saturating concentration of ipsapirone (10 μM). pEC50 values: control = 7.40 ± 0.04 (EC50 = 39 nM); EPPTB = 7.05 ± 0.03 (EC50 = 88 nM); RO5166017 = 7.82 ± 0.04 (EC50 = 15 nM; n = 5). (B) Representative traces of ipsapirone (10 μM)-induced currents in the absence (Control) and presence of EPPTB (10 nM) or RO5166017 (500 nM) followed by application of the 5-HT1A receptor antagonist WAY-100135 (1 μM). EPPTB prevented 5HT1A receptor desensitization and reduced the holding current below the initial baseline (dotted line) when WAY-100135 was added. In contrast, RO5166017 did not significantly reduce 5-HT1A receptor desensitization. (Scale bar: 15 pA per 5 min.) The bar graph quantitatively shows the degree of desensitization rate after continuous ipsapirone application for 15 min [I(t), ratio of current amplitude after 15 min; Imax; peak current amplitude]. Data represent the mean ± SEM (n = 5). ***P < 0.001 vs. control.
Fig. 4.
RO5166017 reverses stress-induced hyperthermia (SIH). (A) In NMRI mice, RO5166017 significantly reversed the SIH (dT) at doses 0.1 and 0.3 mg/kg without affecting basal _T_b (
Fig. S4
). At 1 mg/kg, the decrease of dT was attributed to a nonspecific effect on _T_b (
Fig. S4_A_
). The diagram combines the data of two experiments. ***P < 0.001, **P < 0.01 vs. Veh (n = 8–16 per group). (B) RO5166017 (0.1 mg/kg) failed to decrease dT in Taar1−/− C57BL/6 mice in contrast to their WT littermates. In both genotypes, basal _T_b was not affected (
Fig. S4_A_
). **P < 0.01 vs. WT/Veh (n = 8–10 per group). Numbers on the x axes are oral doses of RO5166017 in mg/kg. Veh, vehicle. All data represent the mean ± SEM.
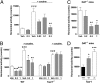
Fig. 5.
RO5166017 blocks hyperlocomotion induced by cocaine or lack of DAT. (A) RO5166017 administered orally dose-dependently reduced cocaine (15 mg/kg i.p.) -induced hyperlocomotion. ###P < 0.001 vs. saline/Veh; **P < 0.01 vs. cocaine/Veh. Cocaine/RO5166017 (0.3 mg/kg) does not differ from groups without cocaine (n = 8 per group). (B) RO5166017 failed to antagonize cocaine (20 mg/kg i.p.) -induced hyperlocomotion in Taar1−/− mice but not in WT littermates. ###P < 0.001 vs. saline groups; ***P < 0.001, **P < 0.01, and *P < 0.05 vs. Veh (n = 10 per group). (C) RO5166017 given i.p. dose-dependently reduced hyperlocomotion in spontaneously hyperactive dopamine transporter knockout (DAT−/−) mice. **P < 0.01, *P < 0.05 vs. Veh (n = 7–8 per group). (D) RO5166017 (0.5 mg/kg i.p.) failed to inhibit hyperlocomotion in DAT−/− mice deficient for Taar1 (−/−) and had reduced effects in DAT−/−/Taar1+/− mice (+/−). +/+, Taar1 WT. ***P < 0.001, *P < 0.05 vs. (+/+) (n = 12–22 per group). Numbers on the x axes are oral doses of RO5166017 in mg/kg. Veh, vehicle. Data represent the mean ± SEM.
Fig. 6.
RO5166017 dose-dependently blocks L-687,414–induced hyperlocomotion. (A) In NMRI mice, RO5166017 administered orally dose-dependently blocked hyperlocomotion triggered by L-687,414 (50 mg/kg s.c.). ###P < 0.001 vs. saline/Veh; **P < 0.01 vs. L-687,414/Veh. L-687,414/RO5166017 (0.1 mg/kg) does not differ from groups without L-687,414 (n = 7–8 per group). (B) In Taar1−/− C57BL/6 mice but not in WT littermates, RO5166017 failed to antagonize hyperlocomotion triggered by L-687,414 (75 mg/kg s.c.). ###P < 0.001 vs. saline/Veh; *P < 0.05 vs. L-687,414/Veh (n = 6–8 per group). Numbers on the x axes are oral doses of RO5166017 in mg/kg. Veh, vehicle. Data represent the mean ± SEM.
Similar articles
- Brain-specific overexpression of trace amine-associated receptor 1 alters monoaminergic neurotransmission and decreases sensitivity to amphetamine.
Revel FG, Meyer CA, Bradaia A, Jeanneau K, Calcagno E, André CB, Haenggi M, Miss MT, Galley G, Norcross RD, Invernizzi RW, Wettstein JG, Moreau JL, Hoener MC. Revel FG, et al. Neuropsychopharmacology. 2012 Nov;37(12):2580-92. doi: 10.1038/npp.2012.109. Epub 2012 Jul 4. Neuropsychopharmacology. 2012. PMID: 22763617 Free PMC article. - Taar1-mediated modulation of presynaptic dopaminergic neurotransmission: role of D2 dopamine autoreceptors.
Leo D, Mus L, Espinoza S, Hoener MC, Sotnikova TD, Gainetdinov RR. Leo D, et al. Neuropharmacology. 2014 Jun;81:283-91. doi: 10.1016/j.neuropharm.2014.02.007. Epub 2014 Feb 22. Neuropharmacology. 2014. PMID: 24565640 - Trace amine-associated receptor 1 partial agonism reveals novel paradigm for neuropsychiatric therapeutics.
Revel FG, Moreau JL, Gainetdinov RR, Ferragud A, Velázquez-Sánchez C, Sotnikova TD, Morairty SR, Harmeier A, Groebke Zbinden K, Norcross RD, Bradaia A, Kilduff TS, Biemans B, Pouzet B, Caron MG, Canales JJ, Wallace TL, Wettstein JG, Hoener MC. Revel FG, et al. Biol Psychiatry. 2012 Dec 1;72(11):934-42. doi: 10.1016/j.biopsych.2012.05.014. Epub 2012 Jun 16. Biol Psychiatry. 2012. PMID: 22705041 - Potential of Ligands for Trace Amine-Associated Receptor 1 (TAAR1) in the Management of Substance Use Disorders.
Wu R, Li JX. Wu R, et al. CNS Drugs. 2021 Dec;35(12):1239-1248. doi: 10.1007/s40263-021-00871-4. Epub 2021 Nov 12. CNS Drugs. 2021. PMID: 34766253 Free PMC article. Review. - Therapeutic Potential of TAAR1 Agonists in Schizophrenia: Evidence from Preclinical Models and Clinical Studies.
Dedic N, Dworak H, Zeni C, Rutigliano G, Howes OD. Dedic N, et al. Int J Mol Sci. 2021 Dec 7;22(24):13185. doi: 10.3390/ijms222413185. Int J Mol Sci. 2021. PMID: 34947997 Free PMC article. Review.
Cited by
- A decade of pharma discovery delivers new tools targeting trace amine-associated receptor 1.
Tallman KR, Grandy DK. Tallman KR, et al. Neuropsychopharmacology. 2012 Nov;37(12):2553-4. doi: 10.1038/npp.2012.148. Neuropsychopharmacology. 2012. PMID: 23070201 Free PMC article. No abstract available. - Discovery of Trace Amine Associated Receptor 1 (TAAR1) Agonist 2-(5-(4'-Chloro-[1,1'-biphenyl]-4-yl)-4_H_-1,2,4-triazol-3-yl)ethan-1-amine (LK00764) for the Treatment of Psychotic Disorders.
Krasavin M, Lukin A, Sukhanov I, Gerasimov AS, Kuvarzin S, Efimova EV, Dorofeikova M, Nichugovskaya A, Matveev A, Onokhin K, Zakharov K, Gureev M, Gainetdinov RR. Krasavin M, et al. Biomolecules. 2022 Nov 7;12(11):1650. doi: 10.3390/biom12111650. Biomolecules. 2022. PMID: 36359001 Free PMC article. - Amphetamine Stimulates Endocytosis of the Norepinephrine and Neuronal Glutamate Transporters in Cultured Locus Coeruleus Neurons.
Underhill SM, Colt MS, Amara SG. Underhill SM, et al. Neurochem Res. 2020 Jun;45(6):1410-1419. doi: 10.1007/s11064-019-02939-6. Epub 2020 Jan 7. Neurochem Res. 2020. PMID: 31912366 Free PMC article. - Trace Amine-Associated Receptor 1 as a Target for the Development of New Antipsychotics: Current Status of Research and Future Directions.
Kantrowitz JT. Kantrowitz JT. CNS Drugs. 2021 Nov;35(11):1153-1161. doi: 10.1007/s40263-021-00864-3. Epub 2021 Oct 15. CNS Drugs. 2021. PMID: 34655036 Review. - Biochemical and Functional Characterization of the Trace Amine-Associated Receptor 1 (TAAR1) Agonist RO5263397.
Espinoza S, Leo D, Sotnikova TD, Shahid M, Kääriäinen TM, Gainetdinov RR. Espinoza S, et al. Front Pharmacol. 2018 Jun 21;9:645. doi: 10.3389/fphar.2018.00645. eCollection 2018. Front Pharmacol. 2018. PMID: 29977204 Free PMC article.
References
- Bunzow JR, et al. Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Mol Pharmacol. 2001;60:1181–1188. - PubMed
- Burchett SA, Hicks TP. The mysterious trace amines: Protean neuromodulators of synaptic transmission in mammalian brain. Prog Neurobiol. 2006;79:223–246. - PubMed
- Berry MD. The potential of trace amines and their receptors for treating neurological and psychiatric diseases. Rev Recent Clin Trials. 2007;2:3–19. - PubMed
- Lindemann L, et al. Trace amine-associated receptors form structurally and functionally distinct subfamilies of novel G protein-coupled receptors. Genomics. 2005;85:372–385. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous