Zinc sparks are triggered by fertilization and facilitate cell cycle resumption in mammalian eggs - PubMed (original) (raw)
. 2011 Jul 15;6(7):716-23.
doi: 10.1021/cb200084y. Epub 2011 Apr 28.
Affiliations
- PMID: 21526836
- PMCID: PMC3171139
- DOI: 10.1021/cb200084y
Zinc sparks are triggered by fertilization and facilitate cell cycle resumption in mammalian eggs
Alison M Kim et al. ACS Chem Biol. 2011.
Abstract
In last few hours of maturation, the mouse oocyte takes up over twenty billion zinc atoms and arrests after the first meiotic division, until fertilization or pharmacological intervention stimulates cell cycle progression toward a new embryo. Using chemical and physical probes, we show that fertilization of the mature, zinc-enriched egg triggers the ejection of zinc into the extracellular milieu in a series of coordinated events termed zinc sparks. These events immediately follow the well-established series of calcium oscillations within the activated egg and are evolutionarily conserved in several mammalian species, including rodents and nonhuman primates. Functionally, the zinc sparks mediate a decrease in intracellular zinc content that is necessary for continued cell cycle progression, as increasing zinc levels within the activated egg results in the reestablishment of cell cycle arrest at metaphase. The mammalian egg thus uses a zinc-dependent switch mechanism to toggle between metaphase arrest and resumption of the meiotic cell cycle at the initiation of embryonic development.
Figures
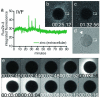
Figure 1. Zinc is released into the extracellular environment as an early fertilization event
Changes in extracellular zinc concentration are readily monitored with FluoZin-3 during in vitro fertilization, and rapid, repetitive increases in fluorescence intensity were detected (a) by ROI analysis (denoted by white boxes in b-d). Each zinc spark can be distinguished in the time-lapse series (b, 00:25:12) against background fluorescence (representative example in c, 01:32:56). Successful fertilization was confirmed by the extrusion of a second polar body (d). Zinc sparks were also noted during strontium chloride-induced parthenogenesis (e, 00:02:56). Time is expressed as hh:mm:ss, wherein 00:00:00 represents the start of image acquisition.
Figure 2. Zinc sparks are polarized and are immediately preceded by intracellular calcium transients
Shortly following activation, zinc sparks occur around the egg cortex with the exception of a zinc spark-free region (a, 00:21:04, arrowhead). This spark-free region corresponds to the region containing the meiotic spindle, where the second polar body is extruded (a, 01:20:00, arrowhead). Egg activation was confirmed by simultaneously monitoring intracellular calcium oscillations with Calcium Green-1 AM every 4 s (b). Intracellular calcium increases immediately before a zinc spark, as evident when images were collected at a faster acquisition rate of every 100 ms in an independent experiment (c). Time is expressed as hh:mm:ss, wherein 00:00:00 represents the start of image acquisition. In all cases, extracellular zinc was detected with FluoZin-3.
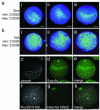
Figure 3. Zinc ions are cortically polarized in the mouse egg
Total zinc, as detected by synchrotron-based x-ray fluorescence (a, b), is uniquely polarized in the unfertilized egg (a; i-iii represent replicates). This distribution is absent in the other essential transition elements, such as iron (b; i-iii represent replicates). The minimum and maximum range of each group of images are given units of μg/cm2. Labile (chelator-accessible) zinc, as detected by confocal microscopy (c-h), also has a hemispherical distribution in the live egg, as detected by two chemically distinct zinc fluorophores: zinquin ethyl ester (c-e) and FluoZin-3 AM (f-h). Co-staining with a DNA marker (Syto 64 in d, Hoechst 33342 in g) revealed that zinc was concentrated at the vegetal pole away from the meiotic spindle. The images depicted here are projected images of the complete Z-series; representative slices from these same Z-stacks confirming cortical localization of zinc are shown in Fig. S5. Scale bar = 20 μm (a, b) or 25 μm (c-h).
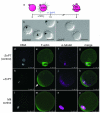
Figure 4. Sustained elevation of intracellular zinc availability following egg activation leads to reestablishment of metaphase arrest
Eggs were activated with strontium chloride (SrCl2) then treated with zinc pyrithione (ZnPT) 1.5 hours later (a). At 6 h post-activation, control eggs form pronuclei (b, arrowheads) whereas the majority of eggs treated with ZnPT do not (c). When visualized by fluorescence, control eggs display decondensed DNA organized within a defined nucleus (d). F-actin is homogeneous around the egg’s cortex (e) and α-tubulin remains organized as a spindle midbody remnant (f), which is visible between the egg and the second polar body (g, merged image). In contrast, ZnPT-treated eggs display condensed chromosomes (h) adjacent to an area of concentrated, cortical F-actin (i). α-tubulin is organized in a metaphase-like configuration (j) around the chromosomes (k, merged image). This layout mirrors the subcellular arrangement in unfertilized eggs (l-o), which also display condensed chromosomes (l) overlaid with an actin cap (m), surrounded by a metaphase spindle (n). Scale bar = 80 μm (b, c) or 25 μm (d-o).
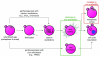
Figure 5. Zinc plays a gatekeeping role, switching between cell cycle arrest and resumption
The unfertilized egg can now be activated by three different approaches, all of them leading to a decrease in intracellular zinc availability. The first is through the physiological pathway, fertilization (center row). This induces calcium oscillations in the egg, leading to the zinc sparks and subsequent cell cycle resumption. The two other approaches induce parthenogenesis and are pharmacological, either using modulators of intracellular calcium (top arrow) or zinc (bottom arrow). The calcium-based approaches induce zinc sparks, while the zinc-based approach bypasses the need for calcium oscillations by directly decreasing the availability of zinc in the egg (green box). Furthermore, cell cycle arrest at metaphase can be reestablished by raising zinc availability (red box), thus implicating zinc as a central regulator of the cell cycle during oocyte maturation and fertilization.
Similar articles
- Bovine eggs release zinc in response to parthenogenetic and sperm-induced egg activation.
Que EL, Duncan FE, Lee HC, Hornick JE, Vogt S, Fissore RA, O'Halloran TV, Woodruff TK. Que EL, et al. Theriogenology. 2019 Mar 15;127:41-48. doi: 10.1016/j.theriogenology.2018.12.031. Epub 2018 Dec 24. Theriogenology. 2019. PMID: 30639695 Free PMC article. - Zinc maintains prophase I arrest in mouse oocytes through regulation of the MOS-MAPK pathway.
Kong BY, Bernhardt ML, Kim AM, O'Halloran TV, Woodruff TK. Kong BY, et al. Biol Reprod. 2012 Jul 1;87(1):11, 1-12. doi: 10.1095/biolreprod.112.099390. Print 2012 Jul. Biol Reprod. 2012. PMID: 22539682 Free PMC article. - Calmodulin-dependent protein kinase gamma 3 (CamKIIgamma3) mediates the cell cycle resumption of metaphase II eggs in mouse.
Chang HY, Minahan K, Merriman JA, Jones KT. Chang HY, et al. Development. 2009 Dec;136(24):4077-81. doi: 10.1242/dev.042143. Epub 2009 Nov 11. Development. 2009. PMID: 19906843 - Transitioning from egg to embryo: triggers and mechanisms of egg activation.
Horner VL, Wolfner MF. Horner VL, et al. Dev Dyn. 2008 Mar;237(3):527-44. doi: 10.1002/dvdy.21454. Dev Dyn. 2008. PMID: 18265018 Review. - Regulation of the meiotic divisions of mammalian oocytes and eggs.
Sanders JR, Jones KT. Sanders JR, et al. Biochem Soc Trans. 2018 Aug 20;46(4):797-806. doi: 10.1042/BST20170493. Epub 2018 Jun 22. Biochem Soc Trans. 2018. PMID: 29934303 Free PMC article. Review.
Cited by
- Zinc Dynamics during Drosophila Oocyte Maturation and Egg Activation.
Hu Q, Duncan FE, Nowakowski AB, Antipova OA, Woodruff TK, O'Halloran TV, Wolfner MF. Hu Q, et al. iScience. 2020 Jul 24;23(7):101275. doi: 10.1016/j.isci.2020.101275. Epub 2020 Jun 16. iScience. 2020. PMID: 32615472 Free PMC article. - eZinCh-2: A Versatile, Genetically Encoded FRET Sensor for Cytosolic and Intraorganelle Zn(2+) Imaging.
Hessels AM, Chabosseau P, Bakker MH, Engelen W, Rutter GA, Taylor KM, Merkx M. Hessels AM, et al. ACS Chem Biol. 2015 Sep 18;10(9):2126-34. doi: 10.1021/acschembio.5b00211. Epub 2015 Jul 20. ACS Chem Biol. 2015. PMID: 26151333 Free PMC article. - A zinc-dependent mechanism regulates meiotic progression in mammalian oocytes.
Bernhardt ML, Kong BY, Kim AM, O'Halloran TV, Woodruff TK. Bernhardt ML, et al. Biol Reprod. 2012 Apr 19;86(4):114. doi: 10.1095/biolreprod.111.097253. Print 2012 Apr. Biol Reprod. 2012. PMID: 22302686 Free PMC article. - Recurrent spontaneous oocyte activation causes female infertility.
Coskun S, Maddirevula S, Awartani K, Aldeery M, Qubbaj W, Kashir J, Alkuraya FS. Coskun S, et al. J Assist Reprod Genet. 2022 Mar;39(3):675-680. doi: 10.1007/s10815-022-02435-x. Epub 2022 Feb 14. J Assist Reprod Genet. 2022. PMID: 35156150 Free PMC article. - Unusual Reactivity and Metal Affinity of Water-Soluble Dipyrrins.
El Khatib M, Cheprakov AV, Vinogradov SA. El Khatib M, et al. Inorg Chem. 2022 Aug 15;61(32):12746-12758. doi: 10.1021/acs.inorgchem.2c01834. Epub 2022 Aug 2. Inorg Chem. 2022. PMID: 35917291 Free PMC article.
References
- Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–29. - PubMed
- Lawrence Y, Whitaker M, Swann K. Sperm-egg fusion is the prelude to the initial Ca2+ increase at fertilization in the mouse. Development. 1997;124:233–41. - PubMed
- Ozil JP, Banrezes B, Toth S, Pan H, Schultz RM. Ca2+ oscillatory pattern in fertilized mouse eggs affects gene expression and development to term. Dev Biol. 2006;300:534–44. - PubMed
- Ducibella T, Huneau D, Angelichio E, Xu Z, Schultz RM, Kopf GS, Fissore R, Madoux S, Ozil JP. Egg-to-embryo transition is driven by differential responses to Ca(2+) oscillation number. Dev Biol. 2002;250:280–91. - PubMed
- Toth S, Huneau D, Banrezes B, Ozil JP. Egg activation is the result of calcium signal summation in the mouse. Reproduction. 2006;131:27–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM038784/GM/NIGMS NIH HHS/United States
- GM038784/GM/NIGMS NIH HHS/United States
- P01 HD021921-19/HD/NICHD NIH HHS/United States
- HD007068/HD/NICHD NIH HHS/United States
- T32 HD007068/HD/NICHD NIH HHS/United States
- R37 GM038784-23/GM/NIGMS NIH HHS/United States
- P01 HD021921/HD/NICHD NIH HHS/United States
- R37 GM038784/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources