Lysyl oxidase contributes to mechanotransduction-mediated regulation of transforming growth factor-β signaling in breast cancer cells - PubMed (original) (raw)
Lysyl oxidase contributes to mechanotransduction-mediated regulation of transforming growth factor-β signaling in breast cancer cells
Molly A Taylor et al. Neoplasia. 2011 May.
Abstract
Transforming growth factor-β (TGF-β) regulates all stages of mammary gland development, including the maintenance of tissue homeostasis and the suppression of tumorigenesis in mammary epithelial cells (MECs). Interestingly, mammary tumorigenesis converts TGF-β from a tumor suppressor to a tumor promoter through molecular mechanisms that remain incompletely understood. Changes in integrin signaling and tissue compliance promote the acquisition of malignant phenotypes in MECs in part through the activity of lysyl oxidase (LOX), which regulates desmoplastic reactions and metastasis. TGF-β also regulates the activities of tumor reactive stroma and MEC metastasis. We show here that TGF-β1 stimulated the synthesis and secretion of LOX from normal and malignant MECs in vitro and in mammary tumors produced in mice. The ability of TGF-β1 to activate Smad2/3 was unaffected by LOX inactivation in normal MECs, whereas the stimulation of p38 MAPK by TGF-β1 was blunted by inhibiting LOX activity in malignant MECs or by inducing the degradation of hydrogen peroxide in both cell types. Inactivating LOX activity impaired TGF-β1-mediated epithelial-mesenchymal transition and invasion in breast cancer cells. We further show that increasing extracellular matrix rigidity by the addition of type I collagen to three-dimensional organotypic cultures promoted the proliferation of malignant MECs, a cellular reaction that was abrogated by inhibiting the activities of TGF-β1 or LOX, and by degrading hydrogen peroxide. Our findings identify LOX as a potential mediator that couples mechanotransduction to oncogenic signaling by TGF-β1 and suggest that measures capable of inactivating LOX function may prove effective in diminishing breast cancer progression stimulated by TGF-β1.
Figures
Figure 1
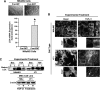
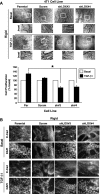
TGF-β1 and EMT stimulate the expression and secretion of LOX in normal and malignant MECs. (A) Shown are representative images of TGF-β1-treated NMuMG cells that have undergone EMT (top panel). TGF-β1 (5 ng/ml) stimulation of EMT in NMuMG cells induced their expression of LOX as determined by semiquantitative real-time PCR. Individual transcript signals were normalized to GAPDH. Data are the mean (±SE; n = 3) fold expression of LOX transcripts relative to pre-EMT NMuMG cells. *P < .05. (B) NMuMG and 4T1 cells were incubated in the absence or presence of TGF-β1 (5 ng/ml) for 24 hours, at which point alterations in the actin cytoskeleton were monitored by TRITC-phalloidin immunofluorescence. Insets: magnified views of boxed regions. Data are representative images from three independent experiments. (C) TGF-β1 (5 ng/ml) stimulated LOX production and secretion from NMuMG and 4T1 cells as determined by immunoblot analysis conditioned medium (C) or detergent-solubilized whole cell extracts (L) with anti-LOX antibodies. Differences in protein loading were monitored by immunoblot analysis for β-actin. Data are representative images from three independent experiments.
Figure 2
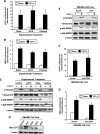
TGF-β1 activates p38 MAPK through a hydrogen peroxide-dependent pathway in normal MECs. NMuMG cells were transiently transfected with the TGF-β1-responsive reporter gene, p3TP-luciferase (A) or pSBE-luciferase (B), and with pCMV-β-gal. Afterward, the transfectants were stimulated with TGF-β1 (5 ng/ml) in the absence or presence of βAPN (300 _µ_M) or catalase (400 U/ml). Luciferase activity was measured and normalized to β-gal. Data are the mean (±SE; n = 3). (C) Quiescent NMuMG cells were pretreated with βAPN (300 µM), hydrogen peroxide (H2O2, 1 mM), or catalase (400 U/ml) as indicated and, subsequently, were stimulated with TGF-β1 (5 ng/ml) for 30 minutes. The phosphorylation and expression levels of Smad2 and p38 MAPK were monitored by immunoblot analysis with phospho-specific antibodies, and differences in protein loading were monitored by reprobing stripped membranes with antibodies against β-actin. Shown are representative images from three independent experiments. (D) Four unique shRNA sequences targeting LOX (sh#1–sh#4) were stably expressed in NMuMG cells and differences in LOX expression were analyzed by immunoblot analysis with anti-LOX antibodies to detect mature LOX. Scram indicates scrambled shRNA. β-Actin immunoreactivity is provided as a loading control. (E) Quiescent scrambled (Scram) and LOX-deficient (sh#4) NMuMG cells were stimulated with TGF-β1 (5 ng/ml) for 30 min, at which point the phosphorylation and expression of Smad2 and p38 MAPK were monitored by immunoblot analysis as described in B. Shown are representative images from three independent experiments. Scrambled (Scram) and LOX-deficient (sh#4) NMuMG cells were transiently transfected with p3TP-luciferase (F) or pSBE-luciferase (G) pCMV-β-gal and, subsequently, were stimulated with TGF-β1 (5 ng/ml) as described in A. Data are the mean (±SE; n = 3).
Figure 3
TGF-β1 regulates EMT in normal MECs through a LOX-and hydrogen peroxide-dependent pathway. (A) NMuMG cells were incubated in the absence or presence of either βAPN (300 _µ_M) or catalase (400 U/ml) while undergoing EMT stimulated by TGF-β1 (5 ng/ml). Arrowheads show strong actin fibers localized to focal adhesions in diluent-treated cells stimulated with TGF-β1 and, conversely, stunted actin fibers in cells treated with βAPN or catalase. (B) Parental (scram) or LOX-deficient (shLOX#3 and shLOX#4) NMuMG cells were stimulated by TGF-β1 (5 ng/ml) to induce EMT. Arrowheads show strong actin fibers localized to focal adhesions in Scram cells stimulated with TGF-β1 and, conversely, the presence of stunted actin fibers in LOX-deficient cells. Shown are representative images from three independent experiments. (C) NMuMG cells were stimulated to undergo EMT by TGF-β1 (5 ng/ml) in the absence (i.e., diluent; Dil) or presence of either βAPN (300 _µ_M) or catalase (400 U/ml; Cat). Altered expression of cytokeratin 19, E-cadherin, N-cadherin, vimentin, or fibronectin mRNA was determined by semiquantitative real-time PCR. Individual transcript signals were normalized to GAPDH. Data are the mean (±SE; n = 3) transcript levels normalized to corresponding unstimulated controls. *P < .05. **P < .05. (D and E) Altered E-cadherin (E-cad) expression was monitored by immunoblot analysis detergent-solubilized whole-cell extracts with anti-E-cadherin antibodies. Protein loading was controlled with anti-β-actin antibodies. Shown are representative images from two independent experiments. Accompanying graphs show the densitometric mean (±SE; n = 2) relative to corresponding basal cells. *P < .05.
Figure 4
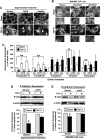
LOX regulates breast cancer cell p38 MAPK activation and invasion stimulated by TGF-β1. (A) Elevated oncogenic TGF-β1 signaling (i.e., WT-TβR-II expression) greatly accelerates the growth and pulmonary metastasis of 4T1 tumors in mice [8]. LOX immunohistochemistry performed on these same tumor slices showed that TGF-β1 signaling significantly induced the expression of LOX expression in WT-TβR-II-expressing 4T1 tumors compared with their GFP-or Y284F-TβR-II-expressing counterparts. Data are representative images from two independent experiments. WT indicates wild-type. 4T1 cells were transiently transfected with p3TP-luciferase (B) or pSBE-luciferase (C) and pCMV-β-gal and, subsequently, were stimulated with TGF-β1 (5 ng/ml) in the absence or presence of βAPN (300 µM) or catalase (400 U/ml). Luciferase activity was measured and normalized to β-gal. Data are the mean (±SE; n = 3). (D) Quiescent 4T1 cells were pretreated with βAPN (300 µM), hydrogen peroxide (H2O2, 1 mM), or catalase (400 U/ml) as indicated and, subsequently, were stimulated with TGF-β1 (5 ng/ml) for 30 minutes. The phosphorylation and expression of Smad2 and p38 MAPK was monitored by immunoblot analysis, and differences in protein loading were monitored by reprobing stripped membranes with antibodies against β-actin. Shown are representative images from three independent experiments. (E) Three unique shRNA sequences targeting LOX (sh#2–sh#4) were stably expressed in 4T1 cells, and differences in LOX expression were analyzed by immunoblot analysis with anti-LOX antibodies. Scram indicates scrambled shRNA. β-Actin immunoreactivity is provided as a loading control. (F) Quiescent scrambled (Scram) and LOX-deficient (sh#4) 4T1 cells were stimulated with TGF-β1 (5 ng/ml) for 30 min, at which point the phosphorylation status and expression of Smad2 and p38 MAPK were monitored by immunoblot analysis as described in D. Shown are representative images from three independent experiments. Scrambled (Scram) and LOX-deficient (sh#4) 4T1 cells were transiently transfected with p3TP-luciferase (G) or pSBE-luciferase (H) and pCMV-β-gal and, subsequently, were stimulated with TGF-β1 (5 ng/ml) as described in A. Data are the mean (±SE; n = 3). (I) 4T1 or MCF10ACA1a (CA1a) cells were incubated in the absence or presence of either βAPN (300 _µ_M) or catalase (400 U/ml) while undergoing invasion through synthetic basement membranes in response to TGF-β1 (5 ng/ml). Data are the mean (±SE; n = 3) invasion relative to that stimulated by TGF-β1. *P < .05.
Figure 5
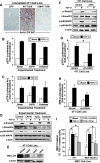
Mechanotransduction induces autocrine TGF-β1 signaling coupled to MEC proliferation. (A) 4T1 cells were in incubated the absence or presence of TGF-β1 (5 ng/ml) in two-dimensional tissue culture plastic or in three-dimensional organotypic cultures supplemented without (i.e., compliant) or with type I collagen (3 mg/ml; rigid). Bright-field images were captured and used to quantitate cell proliferation through ImageJ. Data are the mean (±SE; n = 3) proliferation relative to basal 4T1 cells. *P < .05. (B) Inhibition of TGF-β1 signaling by administration of the TβR-I inhibitor (100 ng/ml) enhanced the growth of 4T1 cells in compliant three-dimensional organotypic cultures, but inhibited their growth in rigid (3 mg/ml type I collagen) three-dimensional organotypic cultures. Insets: magnified views of boxed regions. Data are representative images from three independent experiments. (C) Conditioned medium harvested from compliant or rigid three-dimensional organotypic cultures was acidified to activate total TGF-β1. After sample neutralization, TGF-β1 concentrations were determined by ELISA analysis. Data are the mean (±SE; n = 3) TGF-β1 concentrations relative to those measured in compliant cultures. *P < .05.
Figure 6
Mechanotransduction induces MEC proliferation in a LOX-dependent manner. (A) LOX antagonism using βAPN (300 _µ_M) or catalase (400 U/ml) inhibited the ability of TGF-β1 to stimulate 4T1 cell growth in rigid three-dimensional organotypic cultures. Insets: magnified views of boxed regions. Shown are representative images from three independent experiments. (B) Accompanying data are the mean (±SE; n = 3) proliferation relative to the growth of basal cells in compliant cultures. *P < .05.
Figure 7
LOX deficiency suppresses mechanotransduction and TGF-β1 stimulation of MEC proliferation by restoring cell surface E-cadherin expression. (A) Parental, scrambled (Scram), or LOX-deficient (sh#3 and sh#4) 4T1 cells were propagated in rigid three-dimensional organotypic cultures in the absence or presence of TGF-β1 (5 ng/ml) as indicated. Bright-field images were captured (top panel) and used to quantitate cell proliferation through ImageJ (bottom panel). Insets: magnified views of boxed regions. Data are the mean (±SE; n =3) proliferation relative to basal 4T1 cells. *P < .05. (B) Parental, scrambled (Scram), or LOX-deficient (sh#3 and sh#4) 4T1 cells were propagated in rigid three-dimensional organotypic cultures as described in A and, subsequently, were processed to visualize the expression and localization of E-cadherin by immunofluorescence. Corresponding nuclei were detected by inclusion of DAPI as indicated. Data are representative images from three independent experiments.
Similar articles
- p130Cas is required for mammary tumor growth and transforming growth factor-beta-mediated metastasis through regulation of Smad2/3 activity.
Wendt MK, Smith JA, Schiemann WP. Wendt MK, et al. J Biol Chem. 2009 Dec 4;284(49):34145-56. doi: 10.1074/jbc.M109.023614. Epub 2009 Oct 12. J Biol Chem. 2009. PMID: 19822523 Free PMC article. - Therapeutic targeting of the focal adhesion complex prevents oncogenic TGF-beta signaling and metastasis.
Wendt MK, Schiemann WP. Wendt MK, et al. Breast Cancer Res. 2009;11(5):R68. doi: 10.1186/bcr2360. Breast Cancer Res. 2009. PMID: 19740433 Free PMC article. - The pathophysiology of epithelial-mesenchymal transition induced by transforming growth factor-beta in normal and malignant mammary epithelial cells.
Taylor MA, Parvani JG, Schiemann WP. Taylor MA, et al. J Mammary Gland Biol Neoplasia. 2010 Jun;15(2):169-90. doi: 10.1007/s10911-010-9181-1. Epub 2010 May 15. J Mammary Gland Biol Neoplasia. 2010. PMID: 20467795 Free PMC article. Review. - Noncanonical TGF-β signaling during mammary tumorigenesis.
Parvani JG, Taylor MA, Schiemann WP. Parvani JG, et al. J Mammary Gland Biol Neoplasia. 2011 Jun;16(2):127-46. doi: 10.1007/s10911-011-9207-3. Epub 2011 Mar 31. J Mammary Gland Biol Neoplasia. 2011. PMID: 21448580 Free PMC article. Review.
Cited by
- The interconnectedness of cancer cell signaling.
Rehemtulla A. Rehemtulla A. Neoplasia. 2011 Dec;13(12):1183-93. doi: 10.1593/neo.111746. Neoplasia. 2011. PMID: 22241964 Free PMC article. - Expression Changes and Impact of the Extracellular Matrix on Etoposide Resistant Human Retinoblastoma Cell Lines.
Reinhard J, Wagner N, Krämer MM, Jarocki M, Joachim SC, Dick HB, Faissner A, Kakkassery V. Reinhard J, et al. Int J Mol Sci. 2020 Jun 17;21(12):4322. doi: 10.3390/ijms21124322. Int J Mol Sci. 2020. PMID: 32560557 Free PMC article. - Unraveling the 'TGF-β paradox' one metastamir at a time.
Welch DR, Hurst DR. Welch DR, et al. Breast Cancer Res. 2013 Feb 27;15(1):305. doi: 10.1186/bcr3383. Breast Cancer Res. 2013. PMID: 23448381 Free PMC article. - Lysyl Oxidase Is Predictive of Unfavorable Outcomes and Essential for Regulation of Vascular Endothelial Growth Factor in Hepatocellular Carcinoma.
Zhu J, Huang S, Wu G, Huang C, Li X, Chen Z, Zhao L, Zhao Y. Zhu J, et al. Dig Dis Sci. 2015 Oct;60(10):3019-31. doi: 10.1007/s10620-015-3734-5. Epub 2015 Jun 6. Dig Dis Sci. 2015. PMID: 26048020 - Deptor enhances triple-negative breast cancer metastasis and chemoresistance through coupling to survivin expression.
Parvani JG, Davuluri G, Wendt MK, Espinosa C, Tian M, Danielpour D, Sossey-Alaoui K, Schiemann WP. Parvani JG, et al. Neoplasia. 2015 Mar;17(3):317-28. doi: 10.1016/j.neo.2015.02.003. Neoplasia. 2015. PMID: 25810016 Free PMC article.
References
- Chang CF, Westbrook R, Ma J, Cao D. Transforming growth factor-β signaling in breast cancer. Front Biosci. 2007;12:4393–4401. - PubMed
- Galliher AJ, Neil JR, Schiemann WP. Role of transforming growth factor-β in cancer progression. Future Oncol. 2006;2:743–763. - PubMed
- Moustakas A, Heldin CH. Non-Smad TGF-β signals. J Cell Sci. 2005;118:3573–3584. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical