Transforming growth factor-β2 promotes Snail-mediated endothelial-mesenchymal transition through convergence of Smad-dependent and Smad-independent signalling - PubMed (original) (raw)
Transforming growth factor-β2 promotes Snail-mediated endothelial-mesenchymal transition through convergence of Smad-dependent and Smad-independent signalling
Damian Medici et al. Biochem J. 2011.
Abstract
EndMT (endothelial-mesenchymal transition) is a critical process of cardiac development and disease progression. However, little is know about the signalling mechanisms that cause endothelial cells to transform into mesenchymal cells. In the present paper we show that TGF-β2 (transforming growth factor-β2) stimulates EndMT through the Smad, MEK [MAPK (mitogen-activated protein kinase)/ERK (extracellular-signal-regulated kinase) kinase], PI3K (phosphinositide 3-kinase) and p38 MAPK signalling pathways. Inhibitors of these pathways prevent TGF-β2-induced EndMT. Furthermore, we show that all of these pathways are essential for increasing expression of the cell-adhesion-suppressing transcription factor Snail. Inhibition of Snail with siRNA (small interfering RNA) prevents TGF-β2-induced EndMT. However, overexpression of Snail is not sufficient to cause EndMT. Chemical inhibition of GSK-3β (glycogen synthase kinase-3β) allows EndMT to be induced by Snail overexpression. Expression of a mutant Snail protein that is resistant to GSK-3β-dependent inactivation also promotes EndMT. These results provide the foundation for understanding the roles of specific signalling pathways in mediating EndMT.
© The Authors Journal compilation © 2011 Biochemical Society
Figures
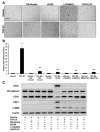
Figure 1. TGF-β2 activates Smad, MEK, PI3K, and p38 MAPK signaling pathways
(A) p3TP-Lux reporter gene assay showing increased Smad activity upon treatment of HCMECs with TGF-β2. Expression of a dominant negative Smad4 (DN-Smad4) inhibited this increased activity. Data represent mean (n=3) ± SD; *P<0.01 for TGF-β2 compared to vehicle; **P<0.05 for TGF-β2 + DN-Smad4 compared to TGF-β2. (B–D) Immunoblotting for phosphorylation levels of ERK1/2 (B), AKT (C), and p38 MAPK (D) showing that TGF-β2 increases phosphorylation of these kinases. Chemical inhibitors against MEK1/2 (U0126; 10μM), PI3K (LY294002; 50μM), and p38 (SB202190; 25μM) inhibit the increases in ERK1/2, AKT, and p38 MAPK phosphorylation, respectively.
Figure 2. TGF-β2 promotes EndMT through Smad-dependent and Smad-independent signaling
(A) DIC imaging showing a change in cell morphology consistent with EndMT in HCMEC cultures treated with TGF-β2. Inhibitors against Smad4 (DN-Smad4), MEK1/2 (U0126; 10μM), PI3K (LY294002; 50μM), or p38 MAPK (SB202190; 25μM) prevented the TGF-β2-induced change in morphology. Scale bar, 20μm. (B) Real-time quantitative PCR analysis showing that TGF-β2 increases Snail gene expression, which is prevented by inhibitors of Smad4, MEK1/2, PI3K, or p38 MAPK. Data represent mean (n=3) ± SD; *P<0.01 for TGF-β2 compared to vehicle; **P<0.01 for all TGF-β2 + inhibitors compared to TGF-β2. (C) Immunoblotting showing that TGF-β2 decreases expression of VE-cadherin and CD31, and increases expression of FSP-1, α-SMA, and Snail. Inhibitors of Smad4, MEK1/2, PI3K, or p38 MAPK prevent these expression changes.
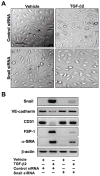
Figure 3. Snail activity is essential for TGF-β2-induced EndMT
(A) DIC imaging showing change in cell morphology in cultures transfected with control siRNA treated with TGF-β2. No EndMT was observed in cultures transfected with Snail siRNA. Scale bar, 20μm. (B) Immunoblotting showing that Snail siRNA inhibits TGF-β2-induced expression changes in VE-cadherin, CD31, FSP-1, and α-SMA.
Figure 4. Snail expression is not sufficient to induce EndMT
(A) DIC imaging showing no effect of snail over-expression on cell morphology. Scale bar, 20μm. (B) Immunoblotting confirming a dramatic increase in Snail gene expression in cells transfected with the Snail expression construct. No significant changes in expression of the endothelial markers VE-cadherin and CD31 or the mesenchymal markers FSP-1 and α-SMA were observed.
Figure 5. Inhibition of GSK-3β allows Snail-induced EndMT
(A) Immunoblotting showing increased phosphorylation of GSK-3β in endothelial cells treated with TGF-β2. Inhibition of PI3K with LY294002 (50μM) is sufficient to block GSK-3β phosphorylation induced by TGF-β1. (B) Immunoblotting demonstrating no phosphorylation of GSK-3β when over-expressing Snail. Lithium chloride (LiCl) is sufficient to induce phosphorylation of GSK-3β in cells transfected with pcDNA3 or pcDNA3-Snail plasmids. Snail expression is increased in cells transfected with pcDNA3-Snail and treated with LiCl. (C) DIC imaging showing that the GSK-3β inhibitor lithium chloride (LiCl) is sufficient to transform endothelial cells transfected with pcDNA3-Snail to mesenchyme. Scale bar, 20μm. (D) Immunoblotting confirming expression of Snail, decreased expression of endothelial markers VE-cadherin and CD31, and increased expression of mesenchymal markers FSP-1 and α-SMA in cells containing pcDNA3-Snail and treated with LiCl.
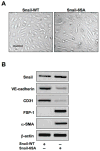
Figure 6. Induction of EndMT by a GSK-3β-resistant mutant form of Snail
(A) DIC imaging demonstrating EndMT of cells transfected with a mutant GSK-3β-resistant Snail (Snail-6SA) construct. Scale bar, 20μm. (B) Immunoblotting showing decreased expression of endothelial markers (VE-cadherin, CD31) and increased expression of mesenchymal markers (FSP-1, α-SMA) in cells expressing mutant Snail, but not wild-type Snail (Snail-WT).
Similar articles
- Semaphorin 7A promotes endothelial to mesenchymal transition through ATF3 mediated TGF-β2/Smad signaling.
Hong L, Li F, Tang C, Li L, Sun L, Li X, Zhu L. Hong L, et al. Cell Death Dis. 2020 Aug 10;11(8):695. doi: 10.1038/s41419-020-02818-x. Cell Death Dis. 2020. PMID: 32826874 Free PMC article. - Blockade of Jagged/Notch pathway abrogates transforming growth factor β2-induced epithelial-mesenchymal transition in human retinal pigment epithelium cells.
Chen X, Xiao W, Liu X, Zeng M, Luo L, Wu M, Ye S, Liu Y. Chen X, et al. Curr Mol Med. 2014 May;14(4):523-34. doi: 10.2174/1566524014666140331230411. Curr Mol Med. 2014. PMID: 24694299 Review. - Isoform-specific effects of transforming growth factor β on endothelial-to-mesenchymal transition.
Sabbineni H, Verma A, Somanath PR. Sabbineni H, et al. J Cell Physiol. 2018 Nov;233(11):8418-8428. doi: 10.1002/jcp.26801. Epub 2018 Jun 1. J Cell Physiol. 2018. PMID: 29856065 Free PMC article. - Foxm1 is a critical driver of TGF-β-induced EndMT in endothelial cells through Smad2/3 and binds to the Snail promoter.
Song S, Zhang R, Cao W, Fang G, Yu Y, Wan Y, Wang C, Li Y, Wang Q. Song S, et al. J Cell Physiol. 2019 Jun;234(6):9052-9064. doi: 10.1002/jcp.27583. Epub 2018 Oct 30. J Cell Physiol. 2019. PMID: 30378114 Free PMC article. - Regulation of endothelial cell plasticity by TGF-β.
van Meeteren LA, ten Dijke P. van Meeteren LA, et al. Cell Tissue Res. 2012 Jan;347(1):177-86. doi: 10.1007/s00441-011-1222-6. Epub 2011 Aug 25. Cell Tissue Res. 2012. PMID: 21866313 Free PMC article. Review.
Cited by
- The essential autophagy gene ATG7 modulates organ fibrosis via regulation of endothelial-to-mesenchymal transition.
Singh KK, Lovren F, Pan Y, Quan A, Ramadan A, Matkar PN, Ehsan M, Sandhu P, Mantella LE, Gupta N, Teoh H, Parotto M, Tabuchi A, Kuebler WM, Al-Omran M, Finkel T, Verma S. Singh KK, et al. J Biol Chem. 2015 Jan 30;290(5):2547-59. doi: 10.1074/jbc.M114.604603. Epub 2014 Dec 19. J Biol Chem. 2015. PMID: 25527499 Free PMC article. - Pathophysiology in Brain Arteriovenous Malformations: Focus on Endothelial Dysfunctions and Endothelial-to-Mesenchymal Transition.
Jeong JY, Bafor AE, Freeman BH, Chen PR, Park ES, Kim E. Jeong JY, et al. Biomedicines. 2024 Aug 7;12(8):1795. doi: 10.3390/biomedicines12081795. Biomedicines. 2024. PMID: 39200259 Free PMC article. Review. - Role of Dlg5/lp-dlg, a membrane-associated guanylate kinase family protein, in epithelial-mesenchymal transition in LLc-PK1 renal epithelial cells.
Sezaki T, Inada K, Sogabe T, Kakuda K, Tomiyama L, Matsuno Y, Ichikawa T, Matsuo M, Ueda K, Kioka N. Sezaki T, et al. PLoS One. 2012;7(4):e35519. doi: 10.1371/journal.pone.0035519. Epub 2012 Apr 23. PLoS One. 2012. PMID: 22539977 Free PMC article. - Eosinophilic Esophagitis: Cytokines Expression and Fibrotic Markers in Comparison to Celiac Disease.
Pronio A, Covotta F, Pallotta L, Palma R, Badiali D, Sacchi MC, Lamazza A, Severi C. Pronio A, et al. Diagnostics (Basel). 2022 Aug 29;12(9):2092. doi: 10.3390/diagnostics12092092. Diagnostics (Basel). 2022. PMID: 36140492 Free PMC article. - Oxidative Stress Enhances the TGF-β2-RhoA-MRTF-A/B Axis in Cells Entering Endothelial-Mesenchymal Transition.
Sobierajska K, Wawro ME, Niewiarowska J. Sobierajska K, et al. Int J Mol Sci. 2022 Feb 13;23(4):2062. doi: 10.3390/ijms23042062. Int J Mol Sci. 2022. PMID: 35216178 Free PMC article.
References
- Boyer AS, Ayerinskas II, Vincent EB, McKinney LA, Weeks DL, Runyan RB. TGFbeta2 and TGFbeta3 have separate and sequential activities during epithelial-mesenchymal cell transformation in the embryonic heart. Dev Biol. 1999;208:530–545. - PubMed
- Camenisch TD, Molin DG, Person A, Runyan RB, Gittenberger-de Groot AC, McDonald JA, Klewer SE. Temporal and distinct TGFbeta ligand requirements during mouse and avian endocardial cushion morphogenesis. Dev Biol. 2002;248:170–181. - PubMed
- Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan X, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–961. - PubMed
- Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 2007;67:10123–10128. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK55001/DK/NIDDK NIH HHS/United States
- U01 CA151925/CA/NCI NIH HHS/United States
- CA151925/CA/NCI NIH HHS/United States
- R01 DK081576/DK/NIDDK NIH HHS/United States
- F30HL095319/HL/NHLBI NIH HHS/United States
- R01 CA125550/CA/NCI NIH HHS/United States
- CA155370/CA/NCI NIH HHS/United States
- F30 HL095319/HL/NHLBI NIH HHS/United States
- CA125550/CA/NCI NIH HHS/United States
- R01 DK055001/DK/NIDDK NIH HHS/United States
- R01 CA155370/CA/NCI NIH HHS/United States
- DK81576/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous