Reduced Mad2 expression keeps relaxed kinetochores from arresting budding yeast in mitosis - PubMed (original) (raw)
Reduced Mad2 expression keeps relaxed kinetochores from arresting budding yeast in mitosis
Erin L Barnhart et al. Mol Biol Cell. 2011.
Abstract
Chromosome segregation depends on the spindle checkpoint, which delays anaphase until all chromosomes have bound microtubules and have been placed under tension. The Mad1-Mad2 complex is an essential component of the checkpoint. We studied the consequences of removing one copy of MAD2 in diploid cells of the budding yeast, Saccharomyces cerevisiae. Compared to MAD2/MAD2 cells, MAD2/mad2Δ heterozygotes show increased chromosome loss and have different responses to two insults that activate the spindle checkpoint: MAD2/mad2Δ cells respond normally to antimicrotubule drugs but cannot respond to chromosomes that lack tension between sister chromatids. In MAD2/mad2Δ cells with normal sister chromatid cohesion, removing one copy of MAD1 restores the checkpoint and returns chromosome loss to wild-type levels. We conclude that cells need the normal Mad2:Mad1 ratio to respond to chromosomes that are not under tension.
Figures
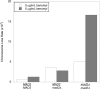
FIGURE 1:
Chromosome loss rates in MAD2/MAD2, _MAD2/mad2_Δ, and _mad2Δ/mad2_Δ diploids. Diploid cells contained a genetically marked chromosome fragment whose loss rates was measured using a colony color-sectoring assay. Measurements were made on plates in the absence (white) or presence (shaded) of 5 μg/ml benomyl.
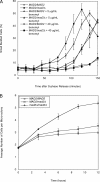
FIGURE 2:
_MAD2/mad2_Δ cells have a mitotic delay in response to microtubule depolymerization. (A) Exponentially dividing _MAD2/MAD2 bub2Δ/bub2_Δ cells and _MAD2/mad2Δ bub2Δ/bub2_Δ cells were arrested in S phase with hydroxyurea. Cells were released into fresh media containing 0, 5, or 40 μg/ml benomyl. The percentage of small, budded cells was determined by light microscopy at the indicated time points. Error bars represent the SD of three separate trials. (B) Exponentially dividing MAD2/MAD2, MAD2/_mad2_Δ, and _mad2Δ/mad2_Δ (all _bub2Δ/bub2_Δ) cells were diluted in rich media (YPD) made with 0.6% low–melting point agar and 60 μg/ml benomyl. The number of cells per microcolony was counted under a light microscope. Error bars represent the SD of three separate trials.
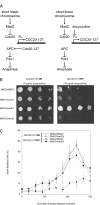
FIGURE 3:
_MAD2/mad2_Δ cells do not arrest in response to short linear chromosomes. (A) A diagram of the effects of short linear chromosomes on the spindle checkpoint. CDC20-127 is a dominant allele of CDC20 that the spindle checkpoint cannot inhibit; in strains in which this allele is expressed from a tetracycline-regulated promoter (Ptet), the response to the spindle checkpoint can be manipulated. In the absence of doxycycline (left) the checkpoint is turned off: CDC20-127 is expressed, activation of the APC is resistant to signals from the checkpoint, and cells execute anaphase and proliferate normally. In contrast, in the presence of doxycycline (right) the checkpoint is turned on: expression of CDC20-127 is repressed, allowing the short linear chromosomes to activate the checkpoint and cause an arrest in mitosis that prevents cell proliferation. (B) Short linear chromosome–dependent activation of the spindle checkpoint. The indicated strains were tested for their ability to proliferate in the absence (CDC20-127 expressed; left) or presence (CDC20-127 repressed; right) of the spindle checkpoint. Serial fourfold dilutions of all strains were spotted onto plates containing 0 μg/ml doxycycline (left) or 10 μg/ml doxycycline (right) and grown for 2 d. (C) _MAD2/mad2_Δ heterozygotes do not have a mitotic delay in response to loss of tension. Exponentially dividing cultures of MAD2/MAD2 and _MAD2/mad2_Δ cells containing Ptet-CDC20-127, CDC28-VF, and short linear chromosomes were arrested in S phase with hydroxyurea. Cells were released into fresh media and treated with 10 μg/ml doxycycline to repress expression of Ptet-CDC20-127, allowing short linear chromosomes to activate the spindle checkpoint. The percentage of small, budded cells after release was determined by light microscopy at the indicated time points. Error bars represent the SD of three separate trials.
FIGURE 4:
_MAD2/mad2_Δ heterozygotes do not arrest in response to a loss of tension on all mitotic chromosomes. We measured cell cycle progression by Western blotting after release from a G1 arrest in the presence or absence of the MCD1, a component of sister chromatid cohesion. Cells were grown to mid-log phase and arrested in G1 with α-factor, and released into glucose to shut off expression of PGAL1-MCD1. Western blots against Myc (top) or actin (bottom) were performed (n = 3). Pds1-18xMyc serves as a marker for metaphase, and a decrease in its level indicates progression into anaphase. Wild-type diploids progress through the cell cycle without delay. In the absence of sister chromatid cohesion, wild-type cells display an extended arrest. However, _MAD2/mad2_Δ and _mad2Δ/mad2_Δ cells do not arrest in response to a loss of tension on all mitotic chromosomes.
FIGURE 5:
_MAD1/mad1Δ MAD2/mad2_Δ cells arrest in response to loss of tension and loss of attachment. (A) Western blots to detect the relative levels of Mad1-TAP (top) or Mad2-TAP (bottom) epitope-tagged strains that are homozygous diploids, heterozygous diploids, or double heterozygous diploids. Both Mad1 and Mad2 proteins levels appear to be lowered by approximately one-half the amount in the heterozygous diploids and double heterozygous diploids relative to the homozygous diploid in comparison to mixed ratios of homozygous TAP-tagged diploids and homozygous nulls (n = 3). (B) The indicated strains were tested for their ability to proliferate in the absence (CDC20-127 expressed; left) or presence (CDC20-127 repressed; right) of the spindle checkpoint. Serial fourfold dilutions of all strains were spotted onto plates containing 0 μg/ml doxycycline (left) or 10 μg/ml doxycycline (right) and grown for 2 d. (C) _MAD1/mad1Δ MAD2/mad2_Δ double heterozygotes have a mitotic delay in response to loss of tension on chromosomes. Error bars represent the SD of three separate trials. (D) _MAD1/mad1Δ MAD2/mad2_Δ double heterozygotes do not arrest in response to a loss of tension on all mitotic chromosomes. We measured cell cycle progression by Western blotting after release from a G1 arrest in the absence of the MCD1. Western blots against Myc (top) or actin (bottom) were performed (n = 3). In the absence of sister chromatid cohesion, wild-type and _MAD1/mad1_Δ cells display an extended arrest. _MAD1/mad1Δ MAD2/mad2_Δ diploid cells do not arrest in response to a loss of cohesion on all mitotic chromosomes.
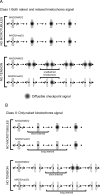
FIGURE 6:
Two classes of models for the spindle checkpoint defect in _MAD2/mad2_Δ cells. (A) Class I. Two types of kinetochores can generate a diffusible signal (shown in gray) that can inhibit the APC: kinetochores that are attached to microtubules but are not under tension (relaxed), and kinetochores that are not attached (naked). Both wild-type MAD2/MAD2 and heterozygous _MAD2/mad2_Δ cells can respond to loss of attachment induced by microtubule depolymerization (top). The relaxed kinetochores can signal in cells that have the normal Mad2:Mad1 ratio but cannot signal in _MAD2/mad2_Δ heterozygotes, whereas the naked kinetochores signal in either cell type. In addition, even though relaxed kinetochores release microtubules, reattachment is faster than release, so most kinetochores remain attached to microtubules. In this scenario, reducing the tension on all the kinetochores cannot arrest a _MAD2/mad2_Δ cell (bottom). (B) class II. Only kinetochores that are not attached to microtubules (naked) can generate a signal that inhibits the APC, and this signal is generated more slowly in _MAD2/mad2_Δ cells. Both wild-type MAD2/MAD2 and heterozygous _MAD2/mad2_Δ cells can respond to loss of attachment induced by microtubule depolymerization (top). In _MAD2/mad2_Δ cells, the reduced Mad2:Mad1 ratio increases the lag before naked kinetochores begin to generate a signal that inhibits the APC and arrests the cell cycle. Without microtubules, the kinetochores cannot reattach, and they eventually generate a signal that arrests the cell cycle (top). However, in the absence of tension when relaxed kinetochores detach from microtubules there is a race between generating the signal that inhibits the APC and reattaching to microtubules (bottom). In MAD2/MAD2 cells, we propose that naked kinetochores can generate a signal that can arrest the cell cycle before they reattach to a microtubule, but the naked kinetochores in _MAD2/mad2_Δ cells reattach to a microtubule before they can generate a signal sufficient to halt the cell cycle (bottom).
Similar articles
- Conserved signalling functions for Mps1, Mad1 and Mad2 in the Cryptococcus neoformans spindle checkpoint.
Aktar K, Davies T, Leontiou I, Clark I, Spanos C, Wallace E, Tuck L, Jeyaprakash AA, Hardwick KG. Aktar K, et al. PLoS Genet. 2024 Jun 3;20(6):e1011302. doi: 10.1371/journal.pgen.1011302. eCollection 2024 Jun. PLoS Genet. 2024. PMID: 38829899 Free PMC article. - Loss of function of the Cik1/Kar3 motor complex results in chromosomes with syntelic attachment that are sensed by the tension checkpoint.
Jin F, Liu H, Li P, Yu HG, Wang Y. Jin F, et al. PLoS Genet. 2012 Feb;8(2):e1002492. doi: 10.1371/journal.pgen.1002492. Epub 2012 Feb 2. PLoS Genet. 2012. PMID: 22319456 Free PMC article. - Lack of tension at kinetochores activates the spindle checkpoint in budding yeast.
Stern BM, Murray AW. Stern BM, et al. Curr Biol. 2001 Sep 18;11(18):1462-7. doi: 10.1016/s0960-9822(01)00451-1. Curr Biol. 2001. PMID: 11566107 - Chromosome segregation in budding yeast: sister chromatid cohesion and related mechanisms.
Marston AL. Marston AL. Genetics. 2014 Jan;196(1):31-63. doi: 10.1534/genetics.112.145144. Genetics. 2014. PMID: 24395824 Free PMC article. Review. - Attachment and tension in the spindle assembly checkpoint.
Zhou J, Yao J, Joshi HC. Zhou J, et al. J Cell Sci. 2002 Sep 15;115(Pt 18):3547-55. doi: 10.1242/jcs.00029. J Cell Sci. 2002. PMID: 12186941 Review.
Cited by
- Deletion of Budding Yeast MAD2 Suppresses Clone-to-Clone Differences in Artificial Linear Chromosome Copy Numbers and Gives Rise to Higher Retention Rates.
Schuyler SC, Wang LI, Ding YS, Lee YC, Chen HY. Schuyler SC, et al. Microorganisms. 2020 Sep 29;8(10):1495. doi: 10.3390/microorganisms8101495. Microorganisms. 2020. PMID: 33003307 Free PMC article. - Phosphorylation of MAD2 at Ser195 Promotes Spindle Checkpoint Defects and Sensitizes Cancer Cells to Radiotherapy in ATM Deficient Cells.
Wang Y, Yu T, Han Y, He Y, Song Y, Guo L, An L, Yang C, Wang F. Wang Y, et al. Front Cell Dev Biol. 2022 Mar 2;10:817831. doi: 10.3389/fcell.2022.817831. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35309941 Free PMC article. - Control of the spindle checkpoint by lateral kinetochore attachment and limited Mad1 recruitment.
Krefman NI, Drubin DG, Barnes G. Krefman NI, et al. Mol Biol Cell. 2015 Jul 15;26(14):2620-39. doi: 10.1091/mbc.E15-05-0276. Epub 2015 May 28. Mol Biol Cell. 2015. PMID: 26023090 Free PMC article. - The Consequences of Chromosome Segregation Errors in Mitosis and Meiosis.
Potapova T, Gorbsky GJ. Potapova T, et al. Biology (Basel). 2017 Feb 8;6(1):12. doi: 10.3390/biology6010012. Biology (Basel). 2017. PMID: 28208750 Free PMC article. Review. - Single-Cell Based Quantitative Assay of Chromosome Transmission Fidelity.
Zhu J, Heinecke D, Mulla WA, Bradford WD, Rubinstein B, Box A, Haug JS, Li R. Zhu J, et al. G3 (Bethesda). 2015 Mar 30;5(6):1043-56. doi: 10.1534/g3.115.017913. G3 (Bethesda). 2015. PMID: 25823586 Free PMC article.
References
- Bardin AJ, Visintin R, Amon A. A mechanism for coupling exit from mitosis to partitioning of the nucleus. Cell. 2000;102:21–31. - PubMed
- Bloecher A, Venturi GM, Tatchell K. Anaphase spindle position is monitored by the BUB2 checkpoint. Nat Cell Biol. 2000;2:556–558. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F32 GM067509/GM/NIGMS NIH HHS/United States
- R01 GM043987/GM/NIGMS NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- GM067509/GM/NIGMS NIH HHS/United States
- GM043987/GM/NIGMS NIH HHS/United States
- R37 GM043987/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases