Molecular mechanisms and clinical applications of angiogenesis - PubMed (original) (raw)
Review
Molecular mechanisms and clinical applications of angiogenesis
Peter Carmeliet et al. Nature. 2011.
Abstract
Blood vessels deliver oxygen and nutrients to every part of the body, but also nourish diseases such as cancer. Over the past decade, our understanding of the molecular mechanisms of angiogenesis (blood vessel growth) has increased at an explosive rate and has led to the approval of anti-angiogenic drugs for cancer and eye diseases. So far, hundreds of thousands of patients have benefited from blockers of the angiogenic protein vascular endothelial growth factor, but limited efficacy and resistance remain outstanding problems. Recent preclinical and clinical studies have shown new molecular targets and principles, which may provide avenues for improving the therapeutic benefit from anti-angiogenic strategies.
Figures
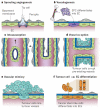
Figure 1. Modes of vessel formation
There are several known methods of blood vessel formation in normal tissues and tumours. a–c, Vessel formation can occur by sprouting angiogenesis (a), by the recruitment of bone-marrow-derived and/or vascular-wall-resident endothelial progenitor cells (EPCs) that differentiate into endothelial cells (ECs; b), or by a process of vessel splitting known as intussusception (c). d–f, Tumour cells can co-opt pre-existing vessels (d), or tumour vessels can be lined by tumour cells (vascular mimicry; e) or by endothelial cells, with cytogenetic abnormalities in their chromosomes, derived from putative cancer stem cells (f). Unlike normal tissues, which use sprouting angiogenesis, vasculogenesis and intussusception (a–c), tumours can use all six modes of vessel formation (a–f).
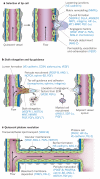
Figure 2. Molecular basis of vessel branching
The consecutive steps of blood vessel branching are shown, with the key molecular players involved denoted in parentheses. a, After stimulation with angiogenic factors, the quiescent vessel dilates and an endothelial cell tip cell is selected (DLL4 and JAGGED1) to ensure branch formation. Tip-cell formation requires degradation of the basement membrane, pericyte detachment and loosening of endothelial cell junctions. Increased permeability permits extravasation of plasma proteins (such as fibrinogen and fibronectin) to deposit a provisional matrix layer, and proteases remodel pre-existing interstitial matrix, all enabling cell migration. For simplicity, only the basement membrane between endothelial cells and pericytes is depicted, but in reality, both pericytes and endothelial cells are embedded in this basement membrane. b, Tip cells navigate in response to guidance signals (such as semaphorins and ephrins) and adhere to the extracellular matrix (mediated by integrins) to migrate. Stalk cells behind the tip cell proliferate, elongate and form a lumen, and sprouts fuse to establish a perfused neovessel. Proliferating stalk cells attract pericytes and deposit basement membranes to become stabilized. Recruited myeloid cells such as tumour-associated macrophages (TAMs) and TIE-2-expressing monocytes (TEMs) can produce pro-angiogenic factors or proteolytically liberate angiogenic growth factors from the ECM. c, After fusion of neighbouring branches, lumen formation allows perfusion of the neovessel, which resumes quiescence by promoting a phalanx phenotype, re-establishment of junctions, deposition of basement membrane, maturation of pericytes and production of vascular maintenance signals. Other factors promote transendothelial lipid transport.
Figure 3. Potential mechanisms of resistance to targeted VEGF therapy in cancer
Different mechanisms underlie the resistance to VEGF blockade seen in some patients with cancer. These mechanisms are not exclusive, and it is likely that several occur simultaneously in a single tumour. a, In established tumours, VEGF blockade aggravates hypoxia, which upregulates the production of other angiogenic factors or increases tumour cell invasiveness. Tumour cells that have acquired other mutations can also become hypoxia tolerant. The more malignant tumour cells are shown as dark green, blue and purple cells. b, Other modes of tumour vascularization, including intussusception, vasculogenic mimicry, differentiation of putative cancer stem cells (CSCs) into endothelial cells (ECs), vasculogenic vessel growth and vessel co-option (all denoted by the mosaic red–purple vessels), may be less sensitive to VEGF blockade. c, Tumour vessels covered by pericytes (green) are less sensitive to VEGF blockade. d, Recruited pro-angiogenic BMDCs (yellow), macrophages (blue and purple) or activated cancer-associated fibroblasts (CAFs; orange) can rescue tumour vascularization by the production of pro-angiogenic factors.
Similar articles
- New insights regarding vessel regression.
Im E, Kazlauskas A. Im E, et al. Cell Cycle. 2006 Sep;5(18):2057-9. doi: 10.4161/cc.5.18.3210. Epub 2006 Sep 15. Cell Cycle. 2006. PMID: 16969103 Review. - A role for VEGF as a negative regulator of pericyte function and vessel maturation.
Greenberg JI, Shields DJ, Barillas SG, Acevedo LM, Murphy E, Huang J, Scheppke L, Stockmann C, Johnson RS, Angle N, Cheresh DA. Greenberg JI, et al. Nature. 2008 Dec 11;456(7223):809-13. doi: 10.1038/nature07424. Epub 2008 Nov 9. Nature. 2008. PMID: 18997771 Free PMC article. - [Angiogenesis - possibilities, problems and perspectives].
Kurzyk A. Kurzyk A. Postepy Biochem. 2015;61(1):25-34. Postepy Biochem. 2015. PMID: 26281351 Review. Polish. - Targeting Angiogenesis in Cancer Therapy: Moving Beyond Vascular Endothelial Growth Factor.
Zhao Y, Adjei AA. Zhao Y, et al. Oncologist. 2015 Jun;20(6):660-73. doi: 10.1634/theoncologist.2014-0465. Epub 2015 May 22. Oncologist. 2015. PMID: 26001391 Free PMC article. Review. - Understanding angiogenesis and the role of angiogenic growth factors in the vascularisation of engineered tissues.
Omorphos NP, Gao C, Tan SS, Sangha MS. Omorphos NP, et al. Mol Biol Rep. 2021 Jan;48(1):941-950. doi: 10.1007/s11033-020-06108-9. Epub 2021 Jan 3. Mol Biol Rep. 2021. PMID: 33393005 Review.
Cited by
- Combination of Ginsenosides Rb2 and Rg3 Promotes Angiogenic Phenotype of Human Endothelial Cells via PI3K/Akt and MAPK/ERK Pathways.
Choi RJ, Mohamad Zobir SZ, Alexander-Dann B, Sharma N, Ma MKL, Lam BYH, Yeo GSH, Zhang W, Fan TP, Bender A. Choi RJ, et al. Front Pharmacol. 2021 Feb 10;12:618773. doi: 10.3389/fphar.2021.618773. eCollection 2021. Front Pharmacol. 2021. PMID: 33643049 Free PMC article. - Endothelial epsin deficiency decreases tumor growth by enhancing VEGF signaling.
Pasula S, Cai X, Dong Y, Messa M, McManus J, Chang B, Liu X, Zhu H, Mansat RS, Yoon SJ, Hahn S, Keeling J, Saunders D, Ko G, Knight J, Newton G, Luscinskas F, Sun X, Towner R, Lupu F, Xia L, Cremona O, De Camilli P, Min W, Chen H. Pasula S, et al. J Clin Invest. 2012 Dec;122(12):4424-38. doi: 10.1172/JCI64537. Epub 2012 Nov 26. J Clin Invest. 2012. PMID: 23187125 Free PMC article. - Myocardial Protection and Current Cancer Therapy: Two Opposite Targets with Inevitable Cost.
Efentakis P, Andreadou I, Iliodromitis KE, Triposkiadis F, Ferdinandy P, Schulz R, Iliodromitis EK. Efentakis P, et al. Int J Mol Sci. 2022 Nov 15;23(22):14121. doi: 10.3390/ijms232214121. Int J Mol Sci. 2022. PMID: 36430599 Free PMC article. Review. - Molecular Mechanism and Approach in Progression of Meningioma.
Shao Z, Liu L, Zheng Y, Tu S, Pan Y, Yan S, Wei Q, Shao A, Zhang J. Shao Z, et al. Front Oncol. 2020 Sep 11;10:538845. doi: 10.3389/fonc.2020.538845. eCollection 2020. Front Oncol. 2020. PMID: 33042832 Free PMC article. Review. - STAT5 and prolactin participate in a positive autocrine feedback loop that promotes angiogenesis.
Yang X, Meyer K, Friedl A. Yang X, et al. J Biol Chem. 2013 Jul 19;288(29):21184-21196. doi: 10.1074/jbc.M113.481119. Epub 2013 Jun 2. J Biol Chem. 2013. PMID: 23729680 Free PMC article.
References
- Folkman J. Angiogenesis: an organizing principle for drug discovery? Nature Rev. Drug Discov. 2007;6:273–286. - PubMed
- Carmeliet P. Angiogenesis in health and disease. Nature Med. 2003;9:653–660. - PubMed
- Swift MR, Weinstein BM. Arterial–venous specification during development. Circ. Res. 2009;104:576–588. - PubMed
- Wang R, et al. Glioblastoma stem-like cells give rise to tumour endothelium. Nature. 2010;468:829–833. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA126642/CA/NCI NIH HHS/United States
- R01 CA085140/CA/NCI NIH HHS/United States
- P01-CA80124/CA/NCI NIH HHS/United States
- R01-CA85140/CA/NCI NIH HHS/United States
- R01 CA115767/CA/NCI NIH HHS/United States
- R01 CA163815/CA/NCI NIH HHS/United States
- R01-CA115767/CA/NCI NIH HHS/United States
- P01 CA080124/CA/NCI NIH HHS/United States
- R01-CA126642/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous