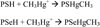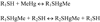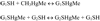Oxidative stress in MeHg-induced neurotoxicity - PubMed (original) (raw)
Review
Oxidative stress in MeHg-induced neurotoxicity
Marcelo Farina et al. Toxicol Appl Pharmacol. 2011.
Abstract
Methylmercury (MeHg) is an environmental toxicant that leads to long-lasting neurological and developmental deficits in animals and humans. Although the molecular mechanisms mediating MeHg-induced neurotoxicity are not completely understood, several lines of evidence indicate that oxidative stress represents a critical event related to the neurotoxic effects elicited by this toxicant. The objective of this review is to summarize and discuss data from experimental and epidemiological studies that have been important in clarifying the molecular events which mediate MeHg-induced oxidative damage and, consequently, toxicity. Although unanswered questions remain, the electrophilic properties of MeHg and its ability to oxidize thiols have been reported to play decisive roles to the oxidative consequences observed after MeHg exposure. However, a close examination of the relationship between low levels of MeHg necessary to induce oxidative stress and the high amounts of sulfhydryl-containing antioxidants in mammalian cells (e.g., glutathione) have led to the hypothesis that nucleophilic groups with extremely high affinities for MeHg (e.g., selenols) might represent primary targets in MeHg-induced oxidative stress. Indeed, the inhibition of antioxidant selenoproteins during MeHg poisoning in experimental animals has corroborated this hypothesis. The levels of different reactive species (superoxide anion, hydrogen peroxide and nitric oxide) have been reported to be increased in MeHg-exposed systems, and the mechanisms concerning these increments seem to involve a complex sequence of cascading molecular events, such as mitochondrial dysfunction, excitotoxicity, intracellular calcium dyshomeostasis and decreased antioxidant capacity. This review also discusses potential therapeutic strategies to counteract MeHg-induced toxicity and oxidative stress, emphasizing the use of organic selenocompounds, which generally present higher affinity for MeHg when compared to the classically studied agents.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
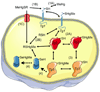
Figure 1. Interaction of MeHg or RSHgMe (a complex of MeHg with a low weight molecular thiol, e.g., cysteine or glutathione) with target cell proteins via exchange reactions
The first event can be of three types: A) MeHg (free MeHg) can react with a target protein (Tg 1) to oxidize it; B) the low-molecular weight complex of MeHg with a thiol-containing molecule (cysteine or small peptides derived from MeHg contaminated fish muscle proteins) can participate in an exchange reaction with the target protein 1 (Tg 1), oxidizing the Tg 1 and regenerating the free thiol molecule; and C) the complex MeHg-cysteine (MeHgSR) can be transported as a mimetic of methionine. The second event can be of two types: A) the MeHg bound to target protein 1 (Tg 1-HgMe complex) can participate in an exchange reaction with a second target protein (Tg 2) to oxidize it and release the free Tg 1; or B) the exchange can occur with intracellular low-molecular- weight thiols (e.g., cysteine or GSH) to form the MeHgSG or MeHgSCys complex and release the free Tg 1. The third event is similar to that described in the second event (i.e., an exchange reaction between a target protein with a different target protein or with intracellular low-molecular thiols). The fourth event represents the tentative unidirectional (indicated by the broken arrows) reactions of intracellular low-molecular MeHg-thiol complexes (RSHgMe) or of a target protein (Tg 3) with a target selenoprotein (Tg 4). In these cases, the selenocysteyl residue of the Tg 4 selenoprotein is oxidized by MeHg and hypothetically cannot participate in an exchange reaction due to the higher affinity of a selenol group for MeHg than that of a thiol group. The aforementioned events (1–4) are represented as numbers (or numbers plus letters) in parentheses (e.g., 1A).
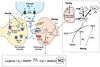
Figure 2. Reactive species as mediators of MeHg induced neurotoxicity
MeHg leads to increased extracellular glutamate (GLU) levels through the inhibition of astrocytic glutamate uptake (event 1), the stimulation of glutamate release from presynaptic terminals (event 2) and the inhibition of vesicular glutamate uptake (event 3). Increased extracellular glutamate levels overactivate _N_-methyl D-aspartate (NMDA)-type glutamate receptors, increasing calcium influx into neurons (event 4). Increased levels of intracellular calcium, which can lead to mitochondrial collapse (event 5), activate neuronal nitric oxide synthase (nNOS) (event 6), thus increasing nitric oxide (NO) formation. MeHg affects the mitochondrial electron transfer chain (mainly at the level of complex II-III) (event 7), leading to the increased formation of superoxide anion (O2•−) and hydrogen peroxide (H2O2). H2O2 can produce hydroxyl radical anion (•OH) via Fenton’s Reaction (event 8). MeHg-induced increases in H2O2 levels might be a consequence of decreased glutathione peroxidase (GPx) activity (event 9) and glutathione (GSH) depletion (event 10).
Scheme 1
Reaction of MeHg (CH3Hg+) with its two main cellular high-molecular generic targets, i.e., thiol- and selenol-containing proteins (PSH and PSeH, respectively).
Scheme 2
Exchange reaction of MeHg from one type of thiol-containing molecule to another class of molecule. R1 and R2 can be either a low- or a high-molecular-weight thiol molecule.
Scheme 3
Exchange reaction of MeHg between two molecules of the same chemical structure, representing the exchange of MeHg between two thiol-containing glutathione, i.e., a reduced glutathione displaces MeHg from a second GS-HgMe complex. Chemically, this reaction indicates a shift of MeHg from one GSH to another GSH molecule. Biologically, this type of exchange reaction is expected to have little significance.
Similar articles
- Protective effects of memantine against methylmercury-induced glutamate dyshomeostasis and oxidative stress in rat cerebral cortex.
Liu W, Xu Z, Deng Y, Xu B, Wei Y, Yang T. Liu W, et al. Neurotox Res. 2013 Oct;24(3):320-37. doi: 10.1007/s12640-013-9386-3. Epub 2013 Mar 16. Neurotox Res. 2013. PMID: 23504438 - Methylmercury-Induced Neurotoxicity: Focus on Pro-oxidative Events and Related Consequences.
Farina M, Aschner M. Farina M, et al. Adv Neurobiol. 2017;18:267-286. doi: 10.1007/978-3-319-60189-2_13. Adv Neurobiol. 2017. PMID: 28889272 Review. - The protective role of tea polyphenols against methylmercury-induced neurotoxic effects in rat cerebral cortex via inhibition of oxidative stress.
Liu W, Xu Z, Yang T, Deng Y, Xu B, Feng S, Li Y. Liu W, et al. Free Radic Res. 2014 Aug;48(8):849-63. doi: 10.3109/10715762.2014.916039. Epub 2014 May 12. Free Radic Res. 2014. PMID: 24821269 - Glutathione antioxidant system and methylmercury-induced neurotoxicity: An intriguing interplay.
Farina M, Aschner M. Farina M, et al. Biochim Biophys Acta Gen Subj. 2019 Dec;1863(12):129285. doi: 10.1016/j.bbagen.2019.01.007. Epub 2019 Jan 16. Biochim Biophys Acta Gen Subj. 2019. PMID: 30659883 Free PMC article. Review. - Oxidative stress accelerates synaptic glutamate dyshomeostasis and NMDARs disorder during methylmercury-induced neuronal apoptosis in rat cerebral cortex.
Yang T, Xu Z, Liu W, Xu B, Deng Y. Yang T, et al. Environ Toxicol. 2020 Jun;35(6):683-696. doi: 10.1002/tox.22904. Epub 2020 Feb 15. Environ Toxicol. 2020. PMID: 32061141
Cited by
- Comparative study on methyl- and ethylmercury-induced toxicity in C6 glioma cells and the potential role of LAT-1 in mediating mercurial-thiol complexes uptake.
Zimmermann LT, Santos DB, Naime AA, Leal RB, Dórea JG, Barbosa F Jr, Aschner M, Rocha JB, Farina M. Zimmermann LT, et al. Neurotoxicology. 2013 Sep;38:1-8. doi: 10.1016/j.neuro.2013.05.015. Epub 2013 May 30. Neurotoxicology. 2013. PMID: 23727015 Free PMC article. - Memantine, a Low-Affinity NMDA Receptor Antagonist, Protects against Methylmercury-Induced Cytotoxicity of Rat Primary Cultured Cortical Neurons, Involvement of Ca2+ Dyshomeostasis Antagonism, and Indirect Antioxidation Effects.
Liu W, Xu Z, Yang T, Xu B, Deng Y, Feng S. Liu W, et al. Mol Neurobiol. 2017 Sep;54(7):5034-5050. doi: 10.1007/s12035-016-0020-2. Epub 2016 Aug 18. Mol Neurobiol. 2017. PMID: 27538940 - Mercury Exposure and Heart Rate Variability: a Systematic Review.
Gribble MO, Cheng A, Berger RD, Rosman L, Guallar E. Gribble MO, et al. Curr Environ Health Rep. 2015 Sep;2(3):304-14. doi: 10.1007/s40572-015-0053-0. Curr Environ Health Rep. 2015. PMID: 26231507 Free PMC article. Review. - Low-dose silver nanoparticles plus methyl mercury exert embryotoxic effects on mouse blastocysts via endoplasmic reticulum stress and mitochondrial apoptosis.
Huang CH, Wang FT, Chan WH. Huang CH, et al. Toxicol Res (Camb). 2022 May 23;11(3):460-474. doi: 10.1093/toxres/tfac028. eCollection 2022 Jun. Toxicol Res (Camb). 2022. PMID: 35782646 Free PMC article. - Postmortem Study of Molecular and Histological Changes in the CA1 Hippocampal Region of Chronic Methamphetamine User.
Mahmoudiasl GR, Abbaszadeh HA, Rezaei-Tavirani M, Abdollahifar MA, Sadeghi Y, Khoramgah MS, Niknazar S, Darabi S. Mahmoudiasl GR, et al. Iran J Pharm Res. 2019 Fall;18(4):2067-2082. doi: 10.22037/ijpr.2019.15483.13123. Iran J Pharm Res. 2019. PMID: 32184870 Free PMC article.
References
- Adams WJ, Jr, Kocsis JJ, Snyder R. Acute toxicity and urinary excretion of diphenyldiselenide. Toxicol Lett. 1989;48:301–310. - PubMed
- Ali SF, LeBel CP, Bondy SC. Reactive oxygen species formation as a biomarker of methylmercury and trimethyltin neurotoxicity. Neurotoxicology. 1992;13:637–648. - PubMed
- Allen JW, Mutkus LA, Aschner M. Methylmercury-mediated inhibition of 3H–D-aspartate transport in cultured astrocytes is reversed by the antioxidant catalase. Brain Res. 2001;902:92–100. - PubMed
- Amonpatumrat S, Sakurai H, Wiriyasermkul P, Khunweeraphong N, Nagamori S, Tanaka H, Piyachaturawat P, Kanai Y. L-glutamate enhances methylmercury toxicity by synergistically increasing oxidative stress. J Pharmacol Sci. 2008;108:280–289. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources