Host defense pathways: role of redundancy and compensation in infectious disease phenotypes - PubMed (original) (raw)
Host defense pathways: role of redundancy and compensation in infectious disease phenotypes
Simone Nish et al. Immunity. 2011.
Abstract
Innate host defense pathways consist of microbial sensors, their signaling pathways, and the antimicrobial effector mechanisms. Several classes of host defense pathways are currently known, each comprising several pattern-recognition receptors that detect different types of pathogens. These pathways interact with one another in a variety of ways that can be categorized into cooperation, complementation, and compensation. Understanding the principles of these interactions is important for better understanding of host defense mechanisms, as well as for correct interpretation of immunodeficient phenotypes.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
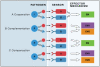
Figure 1. Different types of host pathway interactions
There are three different types of host pathway interactions: cooperation, complementation and compensation. A. Cooperating pathways induce the same effector mechanism more efficiently when engaged simultaneously. B. Complementing pathways induce distinct effector mechanisms (EM1 and EM2), which complement each other to form one functional unit. C. Compensation between two pathways occurs when one pathway is deficient and the other intact. Compensation can take place at the level of sensors (panel C top), or at the level of effectors (panel C, bottom). P – pathogens, A and B – microbial sensors, EM – effector mechanisms.
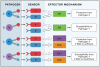
Figure 2. Compensation between host defense pathways and its role in infectious disease phenotypes
Defect in pathways A can be compensated by pathway B in the case of pathogen Px (Panel A), but not in the case of pathogen Py (panel B). Defect in EM1 can be compensated by EM2 if EM2 is sufficient to provide protection against pathogen Px (panel C). If EM2 is not sufficient to protect against Py, then EM2 will not compensate for EM1 deficiency (panel D). Solid lines: intact pathways, dashed lines: inactive pathways. Pathway deficiency can result from either mutations, or due to pathogen evasion.
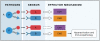
Figure 3. Compensatory enhancement of host defense pathways can result in immunopathology
If more than one pathway is induced by a given infection, they can provide optimal protection with minimal immunopathology, because they do not have to be induced to a maximal level (panel A). If one pathway is deficient, the intact pathway will have to be induced to a higher level, thus increasing the potential for tissue damage.
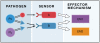
Figure 4
‘Redundancy’ in host defense pathways is conditional on the nature of infection Pathways B is ‘redundant’ when the host is exposed to pathogen Px, but non-redundant when the host is exposed to pathogen Py. Pathways A and B are redundant with regards to the common function (activation of EM1), but non-redundant with regards to activation of EM2. Thus, conclusion of ‘redundancy’ can be affected by both the exposure rates of Px and Py, and by the ‘read-out’ (whether EM2 activation is measured or not).
Similar articles
- Detection of Microbial Infections Through Innate Immune Sensing of Nucleic Acids.
Tan X, Sun L, Chen J, Chen ZJ. Tan X, et al. Annu Rev Microbiol. 2018 Sep 8;72:447-478. doi: 10.1146/annurev-micro-102215-095605. Annu Rev Microbiol. 2018. PMID: 30200854 Review. - [The role of pattern-recognizing receptors in anti-infectious immunity].
Tukhvatulin AI, Shcherbinin DN, Logunov DIu, Shmarov MM, Naroditskiĭ BS. Tukhvatulin AI, et al. Vestn Ross Akad Med Nauk. 2011;(10):47-54. Vestn Ross Akad Med Nauk. 2011. PMID: 22168039 Review. Russian. - Activation of toll-like receptors by microbial lipoproteins: role in host defense.
Modlin RL. Modlin RL. J Allergy Clin Immunol. 2001 Oct;108(4 Suppl):S104-6. doi: 10.1067/mai.2001.118299. J Allergy Clin Immunol. 2001. PMID: 11586275 Review. - Infectious diseases not immune to genome-wide association.
de Bakker PI, Telenti A. de Bakker PI, et al. Nat Genet. 2010 Sep;42(9):731-2. doi: 10.1038/ng0910-731. Nat Genet. 2010. PMID: 20802473 - Pneumonia models and innate immunity to respiratory bacterial pathogens.
Knapp S, Schultz MJ, van der Poll T. Knapp S, et al. Shock. 2005 Dec;24 Suppl 1:12-8. doi: 10.1097/01.shk.0000191385.41689.f3. Shock. 2005. PMID: 16374367 Review.
Cited by
- cAbl Kinase Regulates Inflammasome Activation and Pyroptosis via ASC Phosphorylation.
Gavrilin MA, Prather ER, Vompe AD, McAndrew CC, Wewers MD. Gavrilin MA, et al. J Immunol. 2021 Mar 15;206(6):1329-1336. doi: 10.4049/jimmunol.2000969. Epub 2021 Feb 10. J Immunol. 2021. PMID: 33568399 Free PMC article. - Ensemble modeling of SARS-CoV-2 immune dynamics in immunologically naïve rhesus macaques predicts that potent, early innate immune responses drive viral elimination.
Byrne C, Schiffer JT. Byrne C, et al. Front Immunol. 2024 Nov 7;15:1426016. doi: 10.3389/fimmu.2024.1426016. eCollection 2024. Front Immunol. 2024. PMID: 39575237 Free PMC article. - Detecting the Hidden Properties of Immunological Data and Predicting the Mortality Risks of Infectious Syndromes.
Chatzipanagiotou S, Ioannidis A, Trikka-Graphakos E, Charalampaki N, Sereti C, Piccinini R, Higgins AM, Buranda T, Durvasula R, Hoogesteijn AL, Tegos GP, Rivas AL. Chatzipanagiotou S, et al. Front Immunol. 2016 Jun 10;7:217. doi: 10.3389/fimmu.2016.00217. eCollection 2016. Front Immunol. 2016. PMID: 27375617 Free PMC article. - Control of antiviral immunity by pattern recognition and the microbiome.
Pang IK, Iwasaki A. Pang IK, et al. Immunol Rev. 2012 Jan;245(1):209-26. doi: 10.1111/j.1600-065X.2011.01073.x. Immunol Rev. 2012. PMID: 22168422 Free PMC article. Review. - STAT2 deficiency and susceptibility to viral illness in humans.
Hambleton S, Goodbourn S, Young DF, Dickinson P, Mohamad SM, Valappil M, McGovern N, Cant AJ, Hackett SJ, Ghazal P, Morgan NV, Randall RE. Hambleton S, et al. Proc Natl Acad Sci U S A. 2013 Feb 19;110(8):3053-8. doi: 10.1073/pnas.1220098110. Epub 2013 Feb 7. Proc Natl Acad Sci U S A. 2013. PMID: 23391734 Free PMC article.
References
- Arkwright PD, Abinun M, Cant AJ. Autoimmunity in human primary immunodeficiency diseases. Blood. 2002;99:2694–2702. - PubMed
- Elomaa O, Kangas M, Sahlberg C, Tuukkanen J, Sormunen R, Liakka A, Thesleff I, Kraal G, Tryggvason K. Cloning of a novel bacteria-binding receptor structurally related to scavenger receptors and expressed in a subset of macrophages. Cell. 1995;80:603–609. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI055502/AI/NIAID NIH HHS/United States
- R01 AI055502/AI/NIAID NIH HHS/United States
- R01 AI046688-05/AI/NIAID NIH HHS/United States
- DK071754/DK/NIDDK NIH HHS/United States
- R37 AI046688/AI/NIAID NIH HHS/United States
- R01 AI055502-07S1/AI/NIAID NIH HHS/United States
- R01 AI055502-07/AI/NIAID NIH HHS/United States
- R01 DK071754/DK/NIDDK NIH HHS/United States
- R01 DK071754-05/DK/NIDDK NIH HHS/United States
- R01 AI046688/AI/NIAID NIH HHS/United States
- NIH 055502-07S1/PHS HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical