Zinc homeostasis and signaling in health and diseases: Zinc signaling - PubMed (original) (raw)
Review
Zinc homeostasis and signaling in health and diseases: Zinc signaling
Toshiyuki Fukada et al. J Biol Inorg Chem. 2011 Oct.
Abstract
The essential trace element zinc (Zn) is widely required in cellular functions, and abnormal Zn homeostasis causes a variety of health problems that include growth retardation, immunodeficiency, hypogonadism, and neuronal and sensory dysfunctions. Zn homeostasis is regulated through Zn transporters, permeable channels, and metallothioneins. Recent studies highlight Zn's dynamic activity and its role as a signaling mediator. Zn acts as an intracellular signaling molecule, capable of communicating between cells, converting extracellular stimuli to intracellular signals, and controlling intracellular events. We have proposed that intracellular Zn signaling falls into two classes, early and late Zn signaling. This review addresses recent findings regarding Zn signaling and its role in physiological processes and pathogenesis.
Figures
Fig. 1
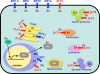
Subcellular localization of zinc (Zn) transporters and metallothioneins (MTs). Localization and potential functions of Zn transporters from the Slc39/Zrt/Irt-like protein (ZIP) (blue) and Slc30/ZnT (red) families, MT, and metal-responsive-element-binding transcription factor 1 (MTF1) within the cell, based on currently available information [, –154]. Arrows show the predicted direction of Zn mobilization. ER endoplasmic reticulum
Fig. 2
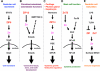
Roles of ZIP and ZnT Zn transporter family members in intracellular signaling. a The signal transducers and activators of transcription 3 (STAT3) downstream target ZIP6 is required for nuclear translocation of the Zn-finger transcription factor Snail, which regulates gastrular cell movement in zebrafish. b ZIP13 is required for the nuclear translocation of Smads in bone morphogenetic protein (BMP)/transforming growth factor beta (TGF-β) signaling, and is involved in tooth, bone, and connective tissue development. c ZIP14, which facilitates G protein-coupled receptor (GPCR) signaling by inhibiting hormone-stimulated phosphodiesterase (PDE) in the pituitary gland, liver, and cartilage, is required for endocrine reactions and systemic growth. d ZnT5 controls protein kinase C (PKC) translocation to the plasma membrane, leading to nuclear factor kappa B (NF-κB)-mediated cytokine production in mast cells under Fc epsilon receptor I (FcεRI) signaling. e Lipopolysaccharide (LPS) stimulation alters the expression of ZIP and ZnT family Zn transporters, resulting in downregulated intracellular Zn levels, followed by dendritic cell maturation and immune responses. TLR Toll-like receptor
Fig. 3
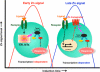
Early and late Zn signaling. Intracellular Zn signaling falls into two types: early Zn signaling (left), in which an extracellular stimulus directly induces elevated Zn levels within several minutes by releasing Zn from a Zn store such as ER or MTs, and late Zn signaling (right), which is induced several hours after stimulation and is dependent on a transcriptional change in Zn transporter expression. Zn waves in mast cells are an example of early Zn signaling (see Fig. 6)
Fig. 4
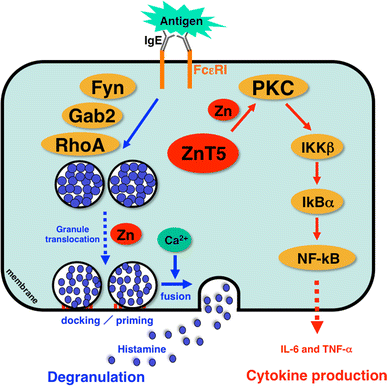
Zn and Zn transporters are indispensable for FcεRI-mediated mast cell activation. Zn is required for multiple steps of FcεRI-induced mast cell activation, including degranulation and cytokine production. Cytosolic Zn regulates the FcεRI-induced granule translocation process, which is mediated by a Fyn/Grb2-associated binder 2 (Gab2)/Ras homologue gene family member A (RhoA)-mediated calcium-independent pathway. Zn and ZnT5 are also required for the translocation of PKC to the plasma membrane and the subsequent nuclear translocation of NF-κB, which leads to the production of cytokines such as interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α). IKKβ I-kappa B kinase beta, IkBα I-kappa B alpha
Fig. 5
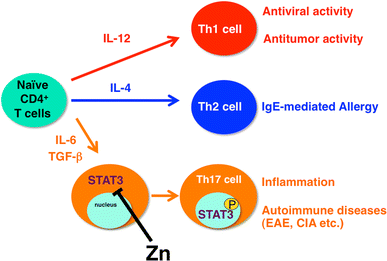
Zn suppresses T helper 17 (Th17) cell development by inhibiting STAT3 activation. Peripheral naive CD4+ T cell precursor cells can differentiate into three subsets of effector T cells (Th1, Th2, and Th17). The differentiation of these subsets is governed by selective cytokines, and each subset accomplishes specialized functions. Th17 cells, which are critical for the development of inflammation and autoimmune disease, are induced by IL-6 and tumor necrosis factor beta (TGF-β). Zn directly binds STAT3, inhibiting its activation by IL-6 and suppressing autoimmune diseases such as experimental autoimmune encephalomyelitis (EAE) and collagen-induced arthritis (CIA). P tyrosine phosphorylation
Fig. 6
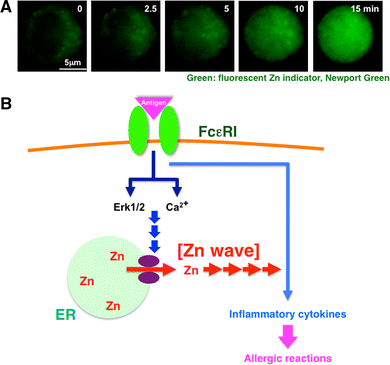
FcεRI increases intracellular free Zn levels, Zn wave: a type of early Zn signaling. a The intracellular Zn level increases within several minutes after antigen stimulation. To monitor the intracellular Zn level, mast cells were treated with the cell-permeable fluorescent Zn indicator Newport Green. Newport Green fluorescence remained steady in the cytoplasm, but gradually increased in the perinuclear and nuclear areas upon FcεRI stimulation. We named this phenomenon a “Zn wave.” b Early Zn signaling in mast cells. Extracellular antigen stimulation induces a Zn wave—a rapid alteration in intracellular Zn level—through the activation of Ca2+ and extracellular-signal-regulated kinase 1 and 2 (Erk1/2) signaling, which might positively affect the signal for inflammatory cytokine-mediated allergic reactions. This suggests that Zn has a role as an intracellular signaling molecule, transducing extracellular stimuli to physiological responses. Violet ovals Zn gatekeepers. (a Modified from Yamasaki et al. [21])
Fig. 7
Model for Zn release for the generation of Zn signaling. a Labile Zn in intracellular Zn storage moves through “Zn gatekeepers” into the cytosol, and approaches target molecules. The Zn gatekeepers are closed in the steady state, but are opened by changes in activation status. b Most of the Zn in the cytosol is sequestered in MTs, and is detached from the MTs by intracellular oxidative stress. The rapid increase in intracellular Zn mediated by cellular events may act as an early Zn signaling to change the status of target molecules
Similar articles
- [Role of zinc transporter in allergic reactions].
Nishida K. Nishida K. Yakugaku Zasshi. 2011 Jan;131(1):85-92. doi: 10.1248/yakushi.131.85. Yakugaku Zasshi. 2011. PMID: 21212618 Review. Japanese. - Zinc: From Biological Functions to Therapeutic Potential.
Costa MI, Sarmento-Ribeiro AB, Gonçalves AC. Costa MI, et al. Int J Mol Sci. 2023 Mar 2;24(5):4822. doi: 10.3390/ijms24054822. Int J Mol Sci. 2023. PMID: 36902254 Free PMC article. Review. - Recent Advances in the Role of SLC39A/ZIP Zinc Transporters In Vivo.
Takagishi T, Hara T, Fukada T. Takagishi T, et al. Int J Mol Sci. 2017 Dec 13;18(12):2708. doi: 10.3390/ijms18122708. Int J Mol Sci. 2017. PMID: 29236063 Free PMC article. Review. - Zinc transporters and signaling in physiology and pathogenesis.
Hojyo S, Fukada T. Hojyo S, et al. Arch Biochem Biophys. 2016 Dec 1;611:43-50. doi: 10.1016/j.abb.2016.06.020. Epub 2016 Jul 6. Arch Biochem Biophys. 2016. PMID: 27394923 Review. - [Zinc signaling-mediated regulation of dentin and periodontal tissues].
Fukada T, Idaira Y, Shimoda S, Asada Y. Fukada T, et al. Clin Calcium. 2015 Dec;25(12):1862-71. Clin Calcium. 2015. PMID: 26608862 Japanese.
Cited by
- Zinc signal: a new player in osteobiology.
Fukada T, Hojyo S, Furuichi T. Fukada T, et al. J Bone Miner Metab. 2013 Mar;31(2):129-35. doi: 10.1007/s00774-012-0409-6. Epub 2012 Nov 27. J Bone Miner Metab. 2013. PMID: 23468210 Review. - Neuronal signalling of zinc: from detection and modulation to function.
Zhang C, Dischler A, Glover K, Qin Y. Zhang C, et al. Open Biol. 2022 Sep;12(9):220188. doi: 10.1098/rsob.220188. Epub 2022 Sep 7. Open Biol. 2022. PMID: 36067793 Free PMC article. Review. - Drug repositioning of polaprezinc for bone fracture healing.
Ko EA, Park YJ, Yoon DS, Lee KM, Kim J, Jung S, Lee JW, Park KH. Ko EA, et al. Commun Biol. 2022 May 16;5(1):462. doi: 10.1038/s42003-022-03424-7. Commun Biol. 2022. PMID: 35577977 Free PMC article. - Zinc and Its Transporters in Epigenetics.
Brito S, Lee MG, Bin BH, Lee JS. Brito S, et al. Mol Cells. 2020 Apr 30;43(4):323-330. doi: 10.14348/molcells.2020.0026. Mol Cells. 2020. PMID: 32274919 Free PMC article. - Increased free Zn2+ correlates induction of sarco(endo)plasmic reticulum stress via altered expression levels of Zn2+ -transporters in heart failure.
Olgar Y, Durak A, Tuncay E, Bitirim CV, Ozcinar E, Inan MB, Tokcaer-Keskin Z, Akcali KC, Akar AR, Turan B. Olgar Y, et al. J Cell Mol Med. 2018 Mar;22(3):1944-1956. doi: 10.1111/jcmm.13480. Epub 2018 Jan 15. J Cell Mol Med. 2018. PMID: 29333637 Free PMC article.
References
- Raulin J. Annales des sciences naturelles. Botanique et biologie végétale. 1869;11:93–345.
- Prasad AS, Halsted JA, Nadimi M. Am J Med. 1961;31:532–546. - PubMed
- Suzuki T, Yoshida N, Suzuki E, Okuda E, Perry AC. Development. 2010;137:2659–2669. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources