Lineage-specific distribution of high levels of genomic 5-hydroxymethylcytosine in mammalian development - PubMed (original) (raw)
. 2011 Sep;21(9):1332-42.
doi: 10.1038/cr.2011.113. Epub 2011 Jul 12.
Yanina Tsenkina, Andrea Serio, Tatiana Dudnakova, Judy Fletcher, Yu Bai, Tatiana Chebotareva, Steve Pells, Zara Hannoun, Gareth Sullivan, Siddharthan Chandran, David C Hay, Mark Bradley, Ian Wilmut, Paul De Sousa
Affiliations
- PMID: 21747414
- PMCID: PMC3193467
- DOI: 10.1038/cr.2011.113
Lineage-specific distribution of high levels of genomic 5-hydroxymethylcytosine in mammalian development
Alexey Ruzov et al. Cell Res. 2011 Sep.
Abstract
Methylation of cytosine is a DNA modification associated with gene repression. Recently, a novel cytosine modification, 5-hydroxymethylcytosine (5-hmC) has been discovered. Here we examine 5-hmC distribution during mammalian development and in cellular systems, and show that the developmental dynamics of 5-hmC are different from those of 5-methylcytosine (5-mC); in particular 5-hmC is enriched in embryonic contexts compared to adult tissues. A detectable 5-hmC signal appears in pre-implantation development starting at the zygote stage, where the paternal genome is subjected to a genome-wide hydroxylation of 5-mC, which precisely coincides with the loss of the 5-mC signal in the paternal pronucleus. Levels of 5-hmC are high in cells of the inner cell mass in blastocysts, and the modification colocalises with nestin-expressing cell populations in mouse post-implantation embryos. Compared to other adult mammalian organs, 5-hmC is strongly enriched in bone marrow and brain, wherein high 5-hmC content is a feature of both neuronal progenitors and post-mitotic neurons. We show that high levels of 5-hmC are not only present in mouse and human embryonic stem cells (ESCs) and lost during differentiation, as has been reported previously, but also reappear during the generation of induced pluripotent stem cells; thus 5-hmC enrichment correlates with a pluripotent cell state. Our findings suggest that apart from the cells of neuronal lineages, high levels of genomic 5-hmC are an epigenetic feature of embryonic cell populations and cellular pluri- and multi-lineage potency. To our knowledge, 5-hmC represents the first epigenetic modification of DNA discovered whose enrichment is so cell-type specific.
© 2011 IBCB, SIBS, CAS All rights reserved
Figures
Figure 1
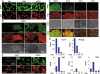
The presence of immunochemically detectable 5-hydroxymethylcytosine (5-hmC) levels correlates with pluripotency in mammalian cells. 5-hmC is detectable in mouse and human (RH1 and RCM1) ESCs. (A) Lines stained with 5-hmC antibody. (B) 5-hmC and 5-mC staining of mouse ESC colonies (defined morphologically on the basis of phase images and indicated by dotted lines) and surrounding fibroblast-like differentiating cells. 5-hmC Immunostaining (C) and quantitative transcriptome deep sequencing (Solexa) data for Oct4, Nanog, Tet1, 2 and 3 (D) of RCM1 cells differentiating towards hepatic endoderm. Identical exposure times were used for different time points. Days post-induction are shown. RPKM – reads per kilobase per million reads. (E) High levels of 5-hmC are detectable in human iPSCs but not in human dermal fibroblasts (HDFs). DNA signal is indicated. Scale bars are 20 μm in A and E, 50 μm in B and 100 μm in C. The experiments were performed using the Diagenode antibody.
Figure 2
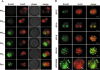
5-Hydroxymethylcytosine (5-hmC) distribution in mouse pre-implantation development. (A) Distribution of 5-hmC and 5-mC during indicated pronuclear stages in mouse zygote. Merged and phase views, where pronuclei are distinguishable at PN3-PN4 stages, are shown. The exposure times were identical for each channel. 5-hmC antibodies produced by Active Motif were used. Scale bar, 10 μm. (B) 5-hmC Distribution at the indicated later stages of mouse pre-implantation development. 5-hmC Antibodies produced by Active Motif were used. 5-mC signal and merge view are indicated. ICM is arrowed. Scale bar, 10 μm.
Figure 3
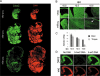
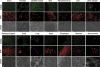
The dynamics of 5-hydroxymethylcytosine (5-hmC) in mouse post-implantational embryonic development. (A) Sagital sections of 10.5 days post coitum (dpc), 12.5 dpc and 15.5 dpc mouse embryos stained with Diagenode antibody to 5-hmC. Immunostaining for single-stranded DNA is shown in red. (B) Fragments of brain and tongue (indicated) embryonic sagital sections at 10.5 dpc, 12.5 dpc and 15.5 dpc stages (indicated). Corresponding slides were immunostained for 5-hmC in parallel with identical conditions and imaged with the same exposure times. Cell populations of embryonic tongue enriched in 5-hmC at 15.5 dpc are arrowed. (C) A schematic showing of the results of quantification of 5-hmC signal in embryonic brain and tongue at 10.5 dpc, 12.5 dpc and 15.5 dpc. Corresponding slides were immunostained for 5-hmC in parallel and imaged with the same exposure times. Mean values for mean signal intensities of 10 random measurements of parts of images shown in B are presented. The same central part of developing brain was quantified for all the stages analysed. Error is expressed as s.e.m. (D) The results of 5-hmC immunostaining of 3 adjacent sections of 12.5 dpc embryo brain region using primary antibody mix pre-incubated with PCR-produced DNA fragments where all the cytosines were replaced with either 5-hmC (5-hmC DNA) or 5-mC (5-mC DNA). Control staining without pre-incubation with any DNA is shown (no DNA). The 5-hmC immunostaining is specifically competed by 5-hmC- but not by 5-mC-containing DNA. Genomic DNA was visualised using single-stranded DNA antibody (indicated) used at a high titre. The experiments were performed using the Diagenode antibody.
Figure 4
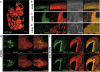
5-Hydroxymethylcytosine (5-hmC) localises with nestin-expressing stem cell populations in 12.5 dpc mouse embryos. (A) Sagital section of 12.5 dpc mouse embryo with DNA visualised and B, C, D, E and F views indicated with dotted squares. Immunohistochemical detection of 5-hmC in sagital sections of mouse embryonic brain (B, C) and heart (D). Localisation of 5-hmC and nestin in the sagital adjacent sections of tailbud (E) and a part of the spinal cord (F). DAPI signal in nestin immunostaining was colourised as red. Anti-single-stranded DNA antibody was used for DNA visualisation in 5-hmC immunostaining experiments. Scale bars are 150 μm in B, C and D and 300 μm in E and F. The experiments were performed using the Diagenode antibody.
Figure 5
The presence of immunochemically detectable 5-hydroxymethylcytosine (5-hmC) levels is restricted to bone marrow and neuronal tissues in adult mammals. A range of indicated normal adult human tissues from a Folio histological tissue array were immunostained with anti-5-hmC antibody. Phase views are shown. An identical maximal exposure of the images made using the 488 nm (5-hmC, tyramide) filter is shown for all the samples, except brain and bone marrow, to illustrate the pattern of background staining, which does not correspond to the DNA signal (indicated) in the majority of tissues. All the triplicate tissue samples on the array exhibited identical staining patterns. Six different bone marrow specimens were tested and gave essentially the same results. Representative views are shown. Scale bars are 20 μm, except 30 μm in bone marrow sample. Essentially the same results were obtained with samples of ovary, testis, kidney, small intestine, salivary gland, uterus, uterine cervix, mesothelium, eye muscle, adrenal gland, pancreas and breast tissue (see Supplementary information, Figure S9). The experiments were performed using the Diagenode antibody.
Figure 6
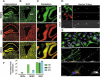
5-Hydroxymethylcytosine (5-hmC) is dramatically enriched in both neuronal progenitors and differentiated post-mitotic cells of neuronal lineages. (A) 5-hmC Is visualised by immunochemistry in hippocampal A and ventricular (B) regions of mouse brain and cerebellar folia (C). Pyramidal layer (pl), dentate gyrus (dg), subventricular zone (svz) and corpus colossum (cc) are indicated. Scale bars are 250 μm. The experiments were performed using the Diagenode antibody. (D) Human neural stem cells (NSCs) exhibit high levels of 5-hmC staining, which does not disappear after 18 days of differentiation into neurons. hESC-derived NSCs (indicated) and neurons derived from them after 18 days (neurons 18 days) of differentiation were immunostained for 5-hmC. 5-hmC, DNA and phase views are indicated. Scale bars are 20 μm. The experiments were performed using the Diagenode antibody. (E) The upper panel shows a representative field of human NSC culture used for 5-hmC immunostaining in D stained for Sox1 (red nuclear signal), DAPI (blue) and nestin (green cytoplasmic signal). The majority of cells co-express both markers. Scale bar is 50 μm. The middle panel shows a representative field of the neuronal culture obtained after 18 days of differentiation and used for 5-hmC immunostaining in D, stained for β-III tubulin (green), Synapsin I (red in the colour image and presented as single channel in the grayscale image on the right) and DAPI (blue). The majority of cells co-express β-III tubulin and Synapsin (scale bar is 50 μm). The lower panel shows the same stained population at a higher magnification (scale bar is 10 μm). (F) The results of real-time RT-PCR analysis of Tet1/2/3 transcripts (indicated) in HDFs, human H1 ES cells and obtained from them human NSCs (indicated). The transcripts of all three Tet genes are highly expressed in human NSCs. The data were normalised with relative to GAPDH. Error is expressed as s.e.m.
Figure 7
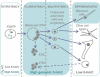
A graphical summary illustrating 5-hydroxymethylcytosine (5-hmC) distribution in mammalian development. High levels of 5-hmC are marked with green shading.
Similar articles
- Array-based assay detects genome-wide 5-mC and 5-hmC in the brains of humans, non-human primates, and mice.
Chopra P, Papale LA, White AT, Hatch A, Brown RM, Garthwaite MA, Roseboom PH, Golos TG, Warren ST, Alisch RS. Chopra P, et al. BMC Genomics. 2014 Feb 13;15:131. doi: 10.1186/1471-2164-15-131. BMC Genomics. 2014. PMID: 24524199 Free PMC article. - High sensitivity 5-hydroxymethylcytosine detection in Balb/C brain tissue.
Davis T, Vaisvila R. Davis T, et al. J Vis Exp. 2011 Feb 1;(48):2661. doi: 10.3791/2661. J Vis Exp. 2011. PMID: 21307836 Free PMC article. - Dynamic regulation of 5-hydroxymethylcytosine in mouse ES cells and during differentiation.
Ficz G, Branco MR, Seisenberger S, Santos F, Krueger F, Hore TA, Marques CJ, Andrews S, Reik W. Ficz G, et al. Nature. 2011 May 19;473(7347):398-402. doi: 10.1038/nature10008. Epub 2011 Apr 3. Nature. 2011. PMID: 21460836 - 5-Hydroxymethylcytosine, the sixth base of the genome.
Münzel M, Globisch D, Carell T. Münzel M, et al. Angew Chem Int Ed Engl. 2011 Jul 11;50(29):6460-8. doi: 10.1002/anie.201101547. Epub 2011 Jun 17. Angew Chem Int Ed Engl. 2011. PMID: 21688365 Review. - 5-hydroxymethylcytosine: a new insight into epigenetics in cancer.
Ye C, Li L. Ye C, et al. Cancer Biol Ther. 2014 Jan;15(1):10-5. doi: 10.4161/cbt.27144. Epub 2013 Nov 19. Cancer Biol Ther. 2014. PMID: 24253310 Free PMC article. Review.
Cited by
- Epigenetic layers and players underlying neurodevelopment.
LaSalle JM, Powell WT, Yasui DH. LaSalle JM, et al. Trends Neurosci. 2013 Aug;36(8):460-70. doi: 10.1016/j.tins.2013.05.001. Epub 2013 May 31. Trends Neurosci. 2013. PMID: 23731492 Free PMC article. Review. - Increased copy number for methylated maternal 15q duplications leads to changes in gene and protein expression in human cortical samples.
Scoles HA, Urraca N, Chadwick SW, Reiter LT, Lasalle JM. Scoles HA, et al. Mol Autism. 2011 Dec 12;2(1):19. doi: 10.1186/2040-2392-2-19. Mol Autism. 2011. PMID: 22152151 Free PMC article. - Early maternal alcohol consumption alters hippocampal DNA methylation, gene expression and volume in a mouse model.
Marjonen H, Sierra A, Nyman A, Rogojin V, Gröhn O, Linden AM, Hautaniemi S, Kaminen-Ahola N. Marjonen H, et al. PLoS One. 2015 May 13;10(5):e0124931. doi: 10.1371/journal.pone.0124931. eCollection 2015. PLoS One. 2015. PMID: 25970770 Free PMC article. - Ontogeny, distribution and potential roles of 5-hydroxymethylcytosine in human liver function.
Ivanov M, Kals M, Kacevska M, Barragan I, Kasuga K, Rane A, Metspalu A, Milani L, Ingelman-Sundberg M. Ivanov M, et al. Genome Biol. 2013 Aug 19;14(8):R83. doi: 10.1186/gb-2013-14-8-r83. Genome Biol. 2013. PMID: 23958281 Free PMC article. - High Performance Liquid Chromatography Separation of Epigenetic Cytosine Variants.
Roberts CE, Raner GM, Isaacs GD. Roberts CE, et al. Methods Protoc. 2018 Mar 21;1(2):10. doi: 10.3390/mps1020010. Methods Protoc. 2018. PMID: 31164555 Free PMC article.
References
- Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6–21. - PubMed
- Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science. 2001;293:1089–1093. - PubMed
- Surani MA, Hayashi K, Hajkova P. Genetic and epigenetic regulators of pluripotency. Cell. 2007;128:747–762. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases