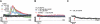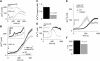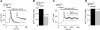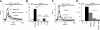Dendritic cell maturation and chemotaxis is regulated by TRPM2-mediated lysosomal Ca2+ release - PubMed (original) (raw)
Dendritic cell maturation and chemotaxis is regulated by TRPM2-mediated lysosomal Ca2+ release
Adriana Sumoza-Toledo et al. FASEB J. 2011 Oct.
Abstract
Chemokines induce calcium (Ca(2+)) signaling and chemotaxis in dendritic cells (DCs), but the molecular players involved in shaping intracellular Ca(2+) changes remain to be characterized. Using siRNA and knockout mice, we show that in addition to inositol 1,4,5-trisphosphate (IP(3))-mediated Ca(2+) release and store-operated Ca(2+) entry (SOCE), the transient receptor potential melastatin 2 (TRPM2) channel contributes to Ca(2+) release but not Ca(2+) influx in mouse DCs. Consistent with these findings, TRPM2 expression in DCs is restricted to endolysosomal vesicles, whereas in neutrophils, the channel localizes to the plasma membrane. TRPM2-deficient DCs show impaired maturation and severely compromised chemokine-activated directional migration as well as bacterial-induced DC trafficking to the draining lymph nodes. Defective DC chemotaxis is due to perturbed chemokine-receptor-initiated Ca(2+) signaling mechanisms, which include suppression of TRPM2-mediated Ca(2+) release and secondary modification of SOCE. DCs deficient in both TRPM2 and IP(3) receptor signaling lose their ability to perform chemotaxis entirely. These results highlight TRPM2 as a key player regulating DC chemotaxis through its function as Ca(2+) release channel and confirm ADP-ribose as a novel second messenger for intracellular Ca(2+) mobilization.
Figures
Figure 1.
Differential expression and function of TRPM2 in mouse phagocytic cells. A) TRPM2 mRNA detection in DC 2.4 cells, BMDCs, PMNs, 3T3 cells, brain cells, and T cells by RT-PCR. Amplification without retrotranscriptase was performed to exclude DNA contamination. GADPH housekeeping gene was used as control. B) Cellular localization of TRPM2 in PMNs and BMDCs. TRPM2 localization was assessed using rabbit anti-human TRPM2 antibody and Alexa Fluor 488-anti-rabbit IgG as secondary antibody. Images are representative of >3 independent experiments. C) Dose-response curve of TRPM2 currents in response to increasing concentrations of intracellular ADPR in mouse PMNs. Cells were kept in standard external solution supplemented with 1 mM Ca2+. Cells were perfused with standard Cs-glutamate-based internal solution supplemented with ADPR as indicated and in the absence of Ca2+ buffers (unbuffered conditions). Data were acquired using a 50-ms voltage ramp from −100 to +100 mV given at 0.5 Hz. Current amplitudes were extracted at −80 mV, normalized to cell size (in pF), averaged, and plotted vs. the respective ADPR concentration (_n_=5–6 for each data point). The half-maximal excitatory concentration (EC50 540 nM) and Hill coefficient (Hill 2) was estimated using a dose-response fit. Error bars =
sem
. D) Average TRPM2 currents in WT PMNs with 10 μM ADPR added to the internal solution (_n_=11; black circles). NMDG+ was applied to distinguish TRPM2 currents from leak (black bar). In PMNs isolated from TRPM2−/− mice, no currents developed with 1 mM ADPR (_n_=10; red circles). WT BMDCs did not develop any TRPM2 currents in response to perfusion with 1 mM ADPR in the internal solution (_n_=6; blue circles). E) Representative I-V relationships of ADPR-induced currents in WT PMNs (black trace; extracted at 44 s), WT PMNs plus NMDG+ (black trace; extracted at 110 s), TRPM2−/− PMNs (red trace; extracted at 50 s) and WT BMDCs (blue trace; extracted at 100 s).
Figure 2.
TRPM2 functions as Ca2+ release channel in mouse DCs but not PMNs. BMDCs and PMNs isolated from WT and TRPM2−/− mice were loaded with 5 μM Fura-2-AM. Calcium mobilization was analyzed using the whole-cell patch-clamp configuration where the standard internal solution was supplemented with various concentrations of agonist/antagonist and 200 μM Fura-2. Red arrow indicates whole-cell break-in. Just before break-in, the standard extracellular solution was switched from 1 mM Ca2+ to 0 Ca2+ to isolate release events. A) Average Ca2+ release in BMDCs induced by perfusion of cells with increasing concentrations of ADPR (_n_=6–8). B) Average inhibition of ADPR-induced (10 μM) Ca2+ release in WT BMDCs by 100 μM 8-Br-ADPR (black trace, _n_=10) and 100 μM AMP (blue trace, _n_=11). Absence of ADPR-induced (1 mM) Ca2+ release in BMDCs isolated from TRPM2−/− mice (red trace, _n_=7). C) Absence of ADPR-induced (100 μM) Ca2+ release in PMNs isolated from WT mice (C57BL/6; _n_=4).
Figure 3.
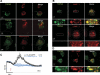
Subcellular localization of TRPM2 is restricted to endolysosomes in DCs. BMDCs were fixed with 2% PFA and permeabilized with 0.2% Triton-X100. TRPM2 localization was assessed using the rabbit anti-human TRPM2 polyclonal antibody, and Alexa Fluor 488-anti-rabbit IgG was used as secondary antibody. A) TRPM2 is not expressed in Golgi, ER, and mitochondria. Cells were costained with 58K Golgi protein (top panel), anti-mouse PDI protein antibody (middle panel) or Mitotracker Red (bottom panel). B) Top panels: immature BMDCs were costained with mouse anti-LAMP1 antibody. Bottom panels: TRPM2−/− BMDCs. C) Immature WT BMDCs were loaded with 5 μM Fura-2-AM for 30 min at 37°C. Average ADPR-induced (10 μM) Ca2+ release in WT BMDCs (black trace, _n_=12), and cells pretreated with 100 nM bafilomycin A1 for 30 min at 37°C (blue trace, _n_=11). 100 nM bafilomycin A1 was also added to the extracellular solution. Ca2+ mobilization was analyzed using patch-clamp. Red arrow indicates whole-cell break-in. Just before break-in, the extracellular solution was switched from 1 mM Ca2+ to 0 Ca2+ to isolate release events. D) TNF-α-matured BMDCs were costained with mouse anti-DC-LAMP. Confocal images are representative of >3 independent experiments. Insets: magnified view of boxed area in corresponding panel.
Figure 4.
Chemokine-induced Ca2+ signaling is altered in TRPM2-deficient DCs. WT and TRPM2−/− BMDCs were selected by CD11c expression at d 3 and used for experiments 24 h later. For mature BMDCs, cells were incubated overnight with 10 ng/ml TNF-α. BMDCs were loaded with 5 μM Fura-2-AM. Error bars =
sem
. A) Representative Ca2+ responses of an immature WT (black trace and dashed trace) and a TRPM2−/− (red trace) BMDC to 300 ng/ml CXCL12 in the presence of 1 mM external Ca2+ (arrow indicates application). B) Average CXCL12-induced Ca2+ response of immature WT (black circles, _n_=56) or TRPM2−/− (red circles, _n_=78) BMDCs. Box magnifies the oscillatory phase during the first 216 s. C) Integral of individual Ca2+ oscillations (see Materials and Methods) assessed during the first 300 s of experimental time in both WT (black bar, _n_=74 spikes) and TRPM2−/− BMDCs (red bar, _n_=28 spikes). D) Representative Ca2+ responses of a mature WT (black trace) and a TRPM2−/− (red trace) BMDC to 75 ng/ml CCL19 in the presence of 1 mM external Ca2+ (arrow indicates application). E) Average CCL19-induced Ca2+ responses in mature WT (black circles, _n_=35) or TRPM2−/− (red circles, _n_=11) BMDCs. F) Average initiation time of the Ca2+ plateau phase in WT (black bar, _n_=30) and TRPM2−/− BMDCs (red bar, _n_=11). Traces were lined up to the cell with lowest basal calcium.
Figure 5.
TRPM2 deficiency differentially affects Ca2+ release depending on DC maturity. BMDCs were isolated and treated as described in Fig. 4. Cells were maintained in standard external solution containing 1 mM Ca2+. Extracellular Ca2+ was removed 1 min before the addition of chemokines (300 ng/ml of CXCL12 or 75 ng/ml of CCL19). Error bars =
sem
. Peak of the first Ca2+ transient measured in each cell was aligned to the earliest peak recorded in each group (immature and mature BMDCs). No difference was found in response time to chemokine between WT and TRPM2−/− immature BMDCs ∼ 200 s. Ca2+ spikes appeared earlier in TRPM2−/− (∼170 s) than in WT (∼200 s) mature BMDCs. A) Average Ca2+ release of immature WT (black circles; _n_=51) and TRPM2−/− BMDCs (red circles; _n_=81) responding with a Ca2+ spike on CXCL12 stimulation (black bar). Inset: data trace from an example WT cell. Arrow indicates chemokine application. B) Averaged Ca2+ integral of the Ca2+ spike in WT BMDCs (black bar, _n_=51) and TRPM2−/− BMDCs (red bar, _n_=81) taken from the data in A. C) Average Ca2+ responses of mature WT (black circles; _n_=96) and TRPM2−/− (red circles; _n_=96) BMDCs with Ca2+ oscillations induced by CCL19 stimulation (black bar). Inset: representative Ca2+ oscillation pattern measured in a WT cell. D) Averaged Ca2+ integral of Ca2+ oscillations measured in individual WT BMDCs (black bar, _n_=916 spikes) and TRPM2−/− BMDCs (red bar, _n_=798 spikes) taken from the data in C. A Ca2+ transient was defined as a peak if it showed an increase and decrease in Ca2+ of ≥10 nM following a normal Gaussian distribution. Graphs represent means of 3 independent experiments. Traces were lined up to the cell with lowest basal calcium.
Figure 6.
TRPM2-deficiency affects DC maturation. A) BMDCs were generated in vitro from WT and TRPM2−/− mice. Representative dot blot graphs indicating the percentage of DCs at d 6 of culture are shown. Samples were stained with APC-CD11c and eFluor 605-CD11b antibodies. B) Time course of DC proliferation of WT and TRPM2−/− BM cultures. C) BMDCs were matured by addition of TNF-α or CpG DNA, and the expression of maturation molecules class-II, CD86, CD80, and CD83 was analyzed by FACS. Changes in the maturation markers were assessed by comparison of TRPM2+/+ BMDC histograms (black line) and TRPM2−/− BMDC (red line) and histogram differences (green line). Statistical significance of the differences was calculated by applying the χ(T) or PB test, wherein a value T(X) > 4 implies that the two distributions are different, with P < 0.01 (99% confidence). T(X) values for TNF-α-treated DCs: class-II, 565; CD80, 990; CD86, 412; and CD83, 379. T(X) values for CPG-treated DCs: class-II, 68; CD80, 1072; CD86, 499; and CD83, 495. Data are from 3 independent experiments (_n_=3). D) Splenic cell suspensions were prepared from WT and TRPM2−/− mice, and samples were stained as in A. Representative dot blot graphs indicating the percentage of CD11b+CD11chi and CD11b+CD11cint DC populations and their total number. E) Splenic samples were stained as in C. Histograms represent mean fluorescence intensity (MFI) of MHC II, CD80, CD86, and CD83 in CD11b+CD11chi (solid bars) and CD11b+CD11cint (open bars) DCs; n = 10. Paired Student's t test was applied for significance analysis; P < 0.05 was considered statistically significant.
Figure 7.
TRPM2 deficiency impairs DC chemotaxis. A) Checkerboard analysis of spontaneous migration (bars 1 and 5), chemoattractant-induced directional migration (bars 2 and 6), or chemokinesis-induced random migration (bars 3, 7, and 4, 8) of immature WT (black bars) or TRPM2−/− (red bars) BMDCs in response to CXCL12 chemokine. Cells were placed into the top wells and chemoattractant was placed in either the top, bottom, or both top and bottom wells of transwell chambers as indicated. Results are expressed as CI of 3 independent experiments (_n_=3). B) Immature and mature WT and TRPM2−/− BMDCs were used at d 5 to test chemotaxis toward medium only, 50 ng/ml CXCL12, or 25 ng/ml CCL19, respectively. Results are expressed as CI of ≥3 independent experiments (_n_=3). C) BMDCs cultured for 5 d were transfected with specific TRPM2 siRNA or scrambled siRNA for 48 h and subjected to TRPM2-immunofluorescence and RT-PCR analysis. D) Chemotaxis assays of immature and mature BMDCs treated with TRPM2 siRNA and scrambled siRNA. Chemotaxis was performed as in A. Results are expressed as CI of 3 independent experiments (_n_=3). E) Immature BMDCs (2×106/50 μl) from EGFP-_trpm2_−/− mice or EGFP-C57BL/6 were injected in the right footpad of 5 C57BL/6 mice 2 h before injection of E. coli (1×106 bacteria/footpad). To detect migratory BMDCs, single-cell suspensions from the right side popliteal and inguinal draining lymph nodes and nondraining lymph nodes (left side controls) were incubated with fluorescent anti-CD11c, anti-MHC class-II, and CD11b and analyzed by flow cytometry. Representative dot blot graphs indicate percentage of CD11c+-GFP MHC+ trpm2+/+ DCs and _trpm2_−/− DCs and total number of migrated cells. Histograms represent total number of migrated BMDCs from 10 mice analyzed in 2 independent experiments. Values are means ±
se
. *P < 0.05; Student's t test.
Figure 8.
Both TRPM2 and IP3R are required for normal Ca2+ release and chemotaxis in DCs. A) Ca2+ measurements in immature WT and TRPM2−/− BMDCs. Cells were loaded with Fluo-3 and then suspended in Hank's buffer without Ca2+/Mg2+. Cells were preincubated for 15 min in the presence or absence of 10 ng/ml bafilomycin A1, and then stimulated with CXCL12 (50 ng/ml). Accumulation of free Ca2+ was measured by FACS over the next 5 min. Arrows indicate intracellular Ca2+ responses of WT, WT + bafilomycin A1, TRPM2−/−, and TRPM2−/− + bafilomycin A1. B) WT or TRPM2−/− immature BMDCs pretreated with bafilomycin A1 (Baf, 100 nM and 1 μM) or concanamycin A (Con A, 1 and 10 μM) or controls were placed in the top chamber of a transwell containing 50 ng/ml CXCL12 and 1.2 mM external Ca2+. Cells that migrated to the bottom chamber in response to the chemotactic gradient were collected and enumerated by FACS. Values represent mean ±
se
CI of triplicate cultures. C) Ca2+ measurements in DCs isolated from WT or TRPM2−/− mice preincubated for 15 min in the presence or absence of the IP3 inhibitor XeC (10 μM), and then stimulated with CXCL12 (50 ng/ml). Free Ca2+ was measured by FACS over the next 5 min. Arrows indicate intracellular Ca2+ responses of WT, TRPM2−/−, and TRPM2−/− cells + XeC. D) Chemotaxis assay as described in B. Cells were pretreated as in C. Values represent mean ±
se
CI of triplicate cultures. Data are representative of 3 independent experiments (_n_=3). ANOVA was applied for significance analysis of CI (B, D) or area under curve (AUC) for the first 80 s (A, C). *P < 0.05.
Similar articles
- TRPM2 functions as a lysosomal Ca2+-release channel in beta cells.
Lange I, Yamamoto S, Partida-Sanchez S, Mori Y, Fleig A, Penner R. Lange I, et al. Sci Signal. 2009 May 19;2(71):ra23. doi: 10.1126/scisignal.2000278. Sci Signal. 2009. PMID: 19454650 Free PMC article. - Akt2- and ETS1-dependent IP3 receptor 2 expression in dendritic cell migration.
Yang W, Nurbaeva MK, Schmid E, Russo A, Almilaji A, Szteyn K, Yan J, Faggio C, Shumilina E, Lang F. Yang W, et al. Cell Physiol Biochem. 2014;33(1):222-36. doi: 10.1159/000356664. Epub 2014 Jan 29. Cell Physiol Biochem. 2014. PMID: 24496246 - ADP-ribose/TRPM2-mediated Ca2+ signaling is essential for cytolytic degranulation and antitumor activity of natural killer cells.
Rah SY, Kwak JY, Chung YJ, Kim UH. Rah SY, et al. Sci Rep. 2015 Mar 25;5:9482. doi: 10.1038/srep09482. Sci Rep. 2015. PMID: 25879940 Free PMC article. - TRPM2: a multifunctional ion channel for calcium signalling.
Sumoza-Toledo A, Penner R. Sumoza-Toledo A, et al. J Physiol. 2011 Apr 1;589(Pt 7):1515-25. doi: 10.1113/jphysiol.2010.201855. Epub 2010 Dec 6. J Physiol. 2011. PMID: 21135052 Free PMC article. Review. - TRPM2: a calcium influx pathway regulated by oxidative stress and the novel second messenger ADP-ribose.
Kühn FJ, Heiner I, Lückhoff A. Kühn FJ, et al. Pflugers Arch. 2005 Oct;451(1):212-9. doi: 10.1007/s00424-005-1446-y. Epub 2005 Jun 11. Pflugers Arch. 2005. PMID: 15952035 Review.
Cited by
- Distribution and Assembly of TRP Ion Channels.
Cheng W, Zheng J. Cheng W, et al. Adv Exp Med Biol. 2021;1349:111-138. doi: 10.1007/978-981-16-4254-8_7. Adv Exp Med Biol. 2021. PMID: 35138613 - TRPM channels: same ballpark, different players, and different rules in immunogenetics.
Farooqi AA, Javeed MK, Javed Z, Riaz AM, Mukhtar S, Minhaj S, Abbas S, Bhatti S. Farooqi AA, et al. Immunogenetics. 2011 Dec;63(12):773-87. doi: 10.1007/s00251-011-0570-4. Epub 2011 Sep 20. Immunogenetics. 2011. PMID: 21932052 Review. - Targeting ion channels for the treatment of autoimmune neuroinflammation.
Bittner S, Meuth SG. Bittner S, et al. Ther Adv Neurol Disord. 2013 Sep;6(5):322-36. doi: 10.1177/1756285613487782. Ther Adv Neurol Disord. 2013. PMID: 23997817 Free PMC article. - Meeting report from the 2nd International Symposium on New Frontiers in Cardiovascular Research. Protecting the cardiovascular system from ischemia: between bench and bedside.
Cabrera-Fuentes HA, Alba-Alba C, Aragones J, Bernhagen J, Boisvert WA, Bøtker HE, Cesarman-Maus G, Fleming I, Garcia-Dorado D, Lecour S, Liehn E, Marber MS, Marina N, Mayr M, Perez-Mendez O, Miura T, Ruiz-Meana M, Salinas-Estefanon EM, Ong SB, Schnittler HJ, Sanchez-Vega JT, Sumoza-Toledo A, Vogel CW, Yarullina D, Yellon DM, Preissner KT, Hausenloy DJ. Cabrera-Fuentes HA, et al. Basic Res Cardiol. 2016 Jan;111(1):7. doi: 10.1007/s00395-015-0527-0. Epub 2015 Dec 14. Basic Res Cardiol. 2016. PMID: 26667317 Free PMC article. - The Antibody Receptor Fc Gamma Receptor IIIb Induces Calcium Entry via Transient Receptor Potential Melastatin 2 in Human Neutrophils.
Alemán OR, Mora N, Rosales C. Alemán OR, et al. Front Immunol. 2021 May 13;12:657393. doi: 10.3389/fimmu.2021.657393. eCollection 2021. Front Immunol. 2021. PMID: 34054821 Free PMC article.
References
- Kennedy A. D., DeLeo F. R. (2009) Neutrophil apoptosis and the resolution of infection. Immunol. Res. 43, 25–61 - PubMed
- Steinman R. M., Banchereau J. (2007) Taking dendritic cells into medicine. Nature 449, 419–426 - PubMed
- Randolph G. J., Ochando J., Partida-Sanchez S. (2008) Migration of dendritic cell subsets and their precursors. Annu. Rev. Immunol. 26, 293–316 - PubMed
- Selvatici R., Falzarano S., Mollica A., Spisani S. (2006) Signal transduction pathways triggered by selective formylpeptide analogues in human neutrophils. Eur. J. Pharmacol. 534, 1–11 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 GM078195/GM/NIGMS NIH HHS/United States
- 2P20RR018727-06/RR/NCRR NIH HHS/United States
- P20-RR016453/RR/NCRR NIH HHS/United States
- G12-RR003061/RR/NCRR NIH HHS/United States
- P20 RR016453/RR/NCRR NIH HHS/United States
- R01AI092117/AI/NIAID NIH HHS/United States
- GM063954/GM/NIGMS NIH HHS/United States
- R01 GM063954/GM/NIGMS NIH HHS/United States
- P20 RR018727/RR/NCRR NIH HHS/United States
- GM078195/GM/NIGMS NIH HHS/United States
- R01 AI092117/AI/NIAID NIH HHS/United States
- G12 RR003061/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous