Effect of the influenza A (H1N1) live attenuated intranasal vaccine on nitric oxide (FE(NO)) and other volatiles in exhaled breath - PubMed (original) (raw)
Comparative Study
Effect of the influenza A (H1N1) live attenuated intranasal vaccine on nitric oxide (FE(NO)) and other volatiles in exhaled breath
A Mashir et al. J Breath Res. 2011 Sep.
Abstract
For the 2009 influenza A (H1N1) pandemic, vaccination and infection control were the main modes of prevention. A live attenuated H1N1 vaccine mimics natural infection and works by evoking a host immune response, but currently there are no easy methods to measure such a response. To determine if an immune response could be measured in exhaled breath, exhaled nitric oxide (FE(NO)) and other exhaled breath volatiles using selected ion flow tube mass spectrometry (SIFT-MS) were measured before and daily for seven days after administering the H1N1 2009 monovalent live intranasal vaccine (FluMist®, MedImmune LLC) in nine healthy healthcare workers (age 35 ± 7 years; five females). On day 3 after H1N1 FluMist® administration there were increases in FE(NO) (MEAN±SEM: day 0 15 ± 3 ppb, day 3 19 ± 3 ppb; p < 0.001) and breath isoprene (MEAN±SEM: day 0 59 ± 15 ppb, day 3 99 ± 17 ppb; p = 0.02). MS analysis identified the greatest number of changes in exhaled breath on day 3 with 137 product ion masses that changed from baseline. The exhaled breath changes on day 3 after H1N1 vaccination may reflect the underlying host immune response. However, further work to elucidate the sources of the exhaled breath changes is necessary.
Figures
Figure 1
(a) SIFT-MS instrument. (b) Exhaled breath collection device consisting of an air filter, flow meter, mouth filter and breath collection bag. Reproduced with permission from Syft Technologies.
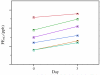
Figure 2
FENO (n = 6) was higher on day 3 compared to baseline (day 0).
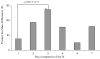
Figure 3
The number of differences in product ion masses from the MS data representing exhaled breath volatiles on days 1–7 after vaccination. The greatest number of product ion masses that changed after baseline (day 0) occurred on day 3.
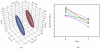
Figure 4
(a) The top three product ion masses identified by discriminant analysis capable of classifying subjects into day 0 or day 3 groups. Normal contour ellipsoids are displayed in red (day 0) on the left, and blue (day 3) on the right. (b) Comparison of one selected product ion mass between day 0 and day 3.
Similar articles
- Influenza virus vaccine live intranasal--MedImmune vaccines: CAIV-T, influenza vaccine live intranasal.
[No authors listed] [No authors listed] Drugs R D. 2003;4(5):312-9. doi: 10.2165/00126839-200304050-00007. Drugs R D. 2003. PMID: 12952502 Review. - Localized mucosal response to intranasal live attenuated influenza vaccine in adults.
Barría MI, Garrido JL, Stein C, Scher E, Ge Y, Engel SM, Kraus TA, Banach D, Moran TM. Barría MI, et al. J Infect Dis. 2013 Jan 1;207(1):115-24. doi: 10.1093/infdis/jis641. Epub 2012 Oct 19. J Infect Dis. 2013. PMID: 23087433 Free PMC article. - Shedding of Ann Arbor strain live attenuated influenza vaccine virus in children 6-59 months of age.
Mallory RM, Yi T, Ambrose CS. Mallory RM, et al. Vaccine. 2011 Jun 10;29(26):4322-7. doi: 10.1016/j.vaccine.2011.04.022. Epub 2011 Apr 20. Vaccine. 2011. PMID: 21513761 Clinical Trial. - [Results of preclinical studies of live monovalent influenza vaccine Influvir].
Mironov AN, Bushmenkov DS, Dyldina NV, Romanova AA, Tsaan AA, Bryzgalova SI, Nikitina OV, Kolbasov SE. Mironov AN, et al. Zh Mikrobiol Epidemiol Immunobiol. 2010 Mar-Apr;(2):35-9. Zh Mikrobiol Epidemiol Immunobiol. 2010. PMID: 20468097 Russian. - Live attenuated intranasal influenza vaccine.
Esposito S, Montinaro V, Groppali E, Tenconi R, Semino M, Principi N. Esposito S, et al. Hum Vaccin Immunother. 2012 Jan;8(1):76-80. doi: 10.4161/hv.8.1.18809. Epub 2012 Jan 1. Hum Vaccin Immunother. 2012. PMID: 22251995 Review.
Cited by
- High and low pathogenicity avian influenza virus discrimination and prediction based on volatile organic compounds signature by SIFT-MS: a proof-of-concept study.
Filaire F, Sécula A, Bessière P, Pagès-Homs M, Guérin JL, Violleau F, Till U. Filaire F, et al. Sci Rep. 2024 Jul 24;14(1):17051. doi: 10.1038/s41598-024-67219-y. Sci Rep. 2024. PMID: 39048690 Free PMC article. - Breath Biomarkers of Influenza Infection.
Danaher PJ, Phillips M, Schmitt P, Richard SA, Millar EV, White BK, Okulicz JF, Coles CL, Burgess TH. Danaher PJ, et al. Open Forum Infect Dis. 2022 Sep 21;9(10):ofac489. doi: 10.1093/ofid/ofac489. eCollection 2022 Oct. Open Forum Infect Dis. 2022. PMID: 36267247 Free PMC article. - Fractional exhaled Nitric Oxide (FeNO) level as a predictor of COVID-19 disease severity.
Lior Y, Yatzkan N, Brami I, Yogev Y, Riff R, Hekselman I, Fremder M, Freixo-Lima G, Be'er M, Amirav I, Lavie M. Lior Y, et al. Nitric Oxide. 2022 Jul 1;124:68-73. doi: 10.1016/j.niox.2022.05.002. Epub 2022 May 18. Nitric Oxide. 2022. PMID: 35597408 Free PMC article. - Using trained dogs and organic semi-conducting sensors to identify asymptomatic and mild SARS-CoV-2 infections: an observational study.
Guest C, Dewhirst SY, Lindsay SW, Allen DJ, Aziz S, Baerenbold O, Bradley J, Chabildas U, Chen-Hussey V, Clifford S, Cottis L, Dennehy J, Foley E, Gezan SA, Gibson T, Greaves CK, Kleinschmidt I, Lambert S, Last A, Morant S, Parker JEA, Pickett J, Quilty BJ, Rooney A, Shah M, Somerville M, Squires C, Walker M, Logan JG; COVID Dogs Research Team. Guest C, et al. J Travel Med. 2022 May 31;29(3):taac043. doi: 10.1093/jtm/taac043. J Travel Med. 2022. PMID: 35325195 Free PMC article. - Breath Metabolites to Diagnose Infection.
Berna AZ, Odom John AR. Berna AZ, et al. Clin Chem. 2021 Dec 30;68(1):43-51. doi: 10.1093/clinchem/hvab218. Clin Chem. 2021. PMID: 34969107 Free PMC article. Review.
References
- Nicholson KG, et al. Clinical studies of monovalent inactivated whole virus and subunit A/USSR/77 (H1N1) vaccine: serological responses and clinical reactions. J. Biol. Stand. 1979;7:123–36. - PubMed
- Mallory RM, Malkin E, Ambrose CS, Bellamy T, Shi L, Yi T, Jones T, Kemble G, Dubovsky F. Safety and immunogenicity following administration of a live, attenuated monovalent 2009 H1N1 influenza vaccine to children and adults in two randomized controlled trials. PLoS ONE. 2010;5:e13755. - PMC - PubMed
- Yang P, et al. Immunogenicity and protective efficacy of a live attenuated vaccine against the 2009 pandemic A H1N1 in mice and ferrets. Vaccine. 2011;29:698–705. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical