Differential utilization of ketone bodies by neurons and glioma cell lines: a rationale for ketogenic diet as experimental glioma therapy - PubMed (original) (raw)
Differential utilization of ketone bodies by neurons and glioma cell lines: a rationale for ketogenic diet as experimental glioma therapy
Gabriele D Maurer et al. BMC Cancer. 2011.
Abstract
Background: Even in the presence of oxygen, malignant cells often highly depend on glycolysis for energy generation, a phenomenon known as the Warburg effect. One strategy targeting this metabolic phenotype is glucose restriction by administration of a high-fat, low-carbohydrate (ketogenic) diet. Under these conditions, ketone bodies are generated serving as an important energy source at least for non-transformed cells.
Methods: To investigate whether a ketogenic diet might selectively impair energy metabolism in tumor cells, we characterized in vitro effects of the principle ketone body 3-hydroxybutyrate in rat hippocampal neurons and five glioma cell lines. In vivo, a non-calorie-restricted ketogenic diet was examined in an orthotopic xenograft glioma mouse model.
Results: The ketone body metabolizing enzymes 3-hydroxybutyrate dehydrogenase 1 and 2 (BDH1 and 2), 3-oxoacid-CoA transferase 1 (OXCT1) and acetyl-CoA acetyltransferase 1 (ACAT1) were expressed at the mRNA and protein level in all glioma cell lines. However, no activation of the hypoxia-inducible factor-1α (HIF-1α) pathway was observed in glioma cells, consistent with the absence of substantial 3-hydroxybutyrate metabolism and subsequent accumulation of succinate. Further, 3-hydroxybutyrate rescued hippocampal neurons from glucose withdrawal-induced cell death but did not protect glioma cell lines. In hypoxia, mRNA expression of OXCT1, ACAT1, BDH1 and 2 was downregulated. In vivo, the ketogenic diet led to a robust increase of blood 3-hydroxybutyrate, but did not alter blood glucose levels or improve survival.
Conclusion: In summary, glioma cells are incapable of compensating for glucose restriction by metabolizing ketone bodies in vitro, suggesting a potential disadvantage of tumor cells compared to normal cells under a carbohydrate-restricted ketogenic diet. Further investigations are necessary to identify co-treatment modalities, e.g. glycolysis inhibitors or antiangiogenic agents that efficiently target non-oxidative pathways.
Figures
Figure 1
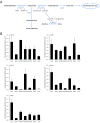
(A) Simplified diagram of cerebral ketone body metabolism. At times of glucose shortage, such as prolonged fasting, ketone bodies are an important energy source for the brain. The oxidoreductase 3-hydroxybutyrate dehydrogenase (BDH) mediates the first step of ketone body degradation, between 3-hydroxybutyrate and acetoacetate. 3-oxoacid-CoA transferase 1 (OXCT1) catalyzes the transfer of coenzyme A from succinyl-CoA to acetoacetate, generating acetoacetyl-CoA. Via acetyl-CoA acetyltransferase 1 (ACAT1), acetoacetyl-CoA is converted into two molecules of acetyl-CoA, which then enter the citric acid cycle. The utilization of ketone bodies results in an elevation of intracellular succinate, leading to HIF-1α stabilization via product inhibition of prolyl hydroxylases (PHD). Furthermore, ketone bodies provide substrates for the synthesis of various molecules, especially lipids. In this regard, acetoacetyl-CoA is formed in the cytoplasm from acetoacetate by the action of acetoacetyl-CoA synthetase (AACS). (B) The expression of the ketone body metabolizing enzymes was analyzed in five glioma cell lines as well as in normal brain (gray, gray matter; white, white matter) by real-time quantitative PCR. Results are presented as the fold change in gene expression normalized to the internal control 18S rRNA (mean and standard deviation; n.d., not detectable).
Figure 2
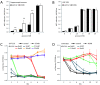
3-hydroxybutyrate protects neonatal rat hippocampal neurons from cell death induced by glucose deprivation. Neonatal rat hippocampal neurons (A) or LNT-229 glioma cells (B) were cultured at different glucose concentrations in the absence (control) or presence of 3-hydroxybutyrate (3OHB, 5 mM). MTT reduction was determined 120 h (hippocampal neurons) or 72 h (LNT-229) after exposure (mean and standard deviation, ** p < 0.01). (C) Glioma cells were grown in medium containing 0 mM, 1 mM, 2.5 mM, 5 mM, 10 mM or 25 mM glucose, supplemented with 3-hydroxybutyrate (3OHB, 5 mM) or not. Cell density was assessed by crystal violet staining at day 1, 2, 3, 4, 6 and 8 after exposure, as shown here for LNT-229 cells (mean and standard deviation). (D) Primary rat astrocytes were treated similarly and crystal violet staining was performed at day 2, 4, 6, 8, 10 and 12 (mean and standard deviation). For a clearer arrangement, 10 mM and 25 mM glucose conditions, showing no difference in cell density between control and 3-hydroxybutyrate supplementation, are not displayed.
Figure 3
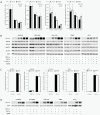
Influence of hypoxia and 3-hydroxybutyrate on the expression of ketone body metabolizing enzymes and the activity of HIF-1α signaling. (A) LNT-229 glioma cells were cultured in the absence or presence of 3-hydroxybutyrate (3OHB, 5 mM) at 21%, 1% or 0.1% oxygen for 24 h. mRNA levels of OXCT1, ACAT1, BDH1 and BDH2 were determined by real-time quantitative PCR. Data are presented as the fold change in gene expression normalized to the internal control 18S rRNA (mean and standard deviation, p < 0.05 compared with normoxic conditions, asterisks omitted for clarity). (B) Glioma cell lines were treated as in (A), and the expression of HIF-1α, BDH1, BDH2, OXCT1 and ACAT1 was analyzed by immunoblot. (C) HIF-specific transcriptional activity was examined by luciferase reporter assay (3HRE-pTK-luc construct) in the absence or presence of 3-hydroxybutyrate (24 h treatment, mean and standard deviation). (D) Glioma cells were incubated for 24 h at the indicated oxygen conditions in the absence or presence of 3-nitropropionic acid (3NPA, 10 mM), and the expression of HIF-1α was analyzed by immunoblot.
Figure 4
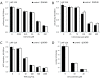
The presence of 3-hydroxybutyrate does not modify the sensitivity of LNT-229 cells to inhibitors of oxidative phosphorylation, glycolysis, TRAIL or temozolomide. LNT-229 cells (mean and standard deviation) were treated with increasing concentrations of (A) rotenone (48 h), (B) 3-bromopyruvate (150 min) or (C) TRAIL (24 h). In the 3-bromopyruvate experiments, cells were preincubated in medium containing 3-hydroxybutyrate (3OHB, 5 mM) or not. Cell density was evaluated by crystal violet staining (A, B) or MTT reduction (C). (D) LNT-229 cells were exposed to temozolomide for 24 h, followed by further observation in drug-free medium supplemented with 3-hydroxybutyrate (3OHB, 5 mM) or not, and clonogenic survival was analyzed (mean and standard deviation).
Figure 5
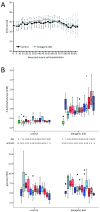
Ketogenic diet induces ketosis but does not lower blood glucose levels. LNT-229 cells were implanted into the right striatum of nude mice at day 0. Thereafter, animals were fed either the standard diet or the ketogenic diet. (A) Body weight was measured twice weekly and is presented as mean and standard deviation. (B) Blood levels of 3-hydroxybutyrate and glucose were determined on the day of tumor cell implantation (day 0) and every 7 days thereafter. 3-hydroxybutyrate values in mice fed the ketogenic diet were significantly higher than those in the control group (Bonferroni-adjusted p < 0.05 at all time points after diet change). By contrast, glucose levels did not differ significantly between diet groups. Boxplots depict the median, quartiles and extreme values. Upper and lower whiskers correspond to the highest and lowest values which are not greater than 1.5 times the interquartile range; •, cases with values between 1.5 and 3 times the interquartile range; *, cases with values more than 3 times the interquartile range.
Figure 6
Ketogenic diet fed ad libitum does not prolong survival in the LNT-229 xenograft model. (A) Mice carrying LNT-229 xenografts were fed with ketogenic or control diet, observed in daily intervals and killed at the onset of neurological symptoms equal or worse than grade 2 (Kaplan-Meier survival estimate, Mantel-Cox log-rank p = 0.288). On days 37 and 65, magnetic resonance imaging of three randomly chosen animals from each group was performed. Representative images (day 65) are depicted. (B) Tumors of mice fed the ketogenic diet displayed proliferation (Ki-67 labeling) indices similar to those of control animals (mean and standard error of the mean, Student's t-test p = 0.637).
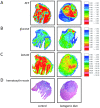
Figure 7
Metabolic mapping does not reveal significant differences between the two diet groups. Adjacent cryosections were used for hematoxylin-eosin staining (D) and for bioluminescence imaging of ATP (A), glucose (B) and lactate (C). Concentration distributions of these metabolites are color-coded [μmol/g].
Similar articles
- A ketogenic diet increases transport and oxidation of ketone bodies in RG2 and 9L gliomas without affecting tumor growth.
De Feyter HM, Behar KL, Rao JU, Madden-Hennessey K, Ip KL, Hyder F, Drewes LR, Geschwind JF, de Graaf RA, Rothman DL. De Feyter HM, et al. Neuro Oncol. 2016 Aug;18(8):1079-87. doi: 10.1093/neuonc/now088. Epub 2016 May 3. Neuro Oncol. 2016. PMID: 27142056 Free PMC article. - Low ketolytic enzyme levels in tumors predict ketogenic diet responses in cancer cell lines in vitro and in vivo.
Zhang J, Jia PP, Liu QL, Cong MH, Gao Y, Shi HP, Yu WN, Miao MY. Zhang J, et al. J Lipid Res. 2018 Apr;59(4):625-634. doi: 10.1194/jlr.M082040. Epub 2018 Feb 5. J Lipid Res. 2018. PMID: 29414764 Free PMC article. - Ketone-body metabolism in glioma and neuroblastoma cells.
Patel MS, Russell JJ, Gershman H. Patel MS, et al. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7214-8. doi: 10.1073/pnas.78.11.7214. Proc Natl Acad Sci U S A. 1981. PMID: 6118869 Free PMC article. - Activation of G protein-coupled receptors by ketone bodies: Clinical implication of the ketogenic diet in metabolic disorders.
Spigoni V, Cinquegrani G, Iannozzi NT, Frigeri G, Maggiolo G, Maggi M, Parello V, Dei Cas A. Spigoni V, et al. Front Endocrinol (Lausanne). 2022 Oct 20;13:972890. doi: 10.3389/fendo.2022.972890. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36339405 Free PMC article. Review. - Ketone body metabolism and its defects.
Fukao T, Mitchell G, Sass JO, Hori T, Orii K, Aoyama Y. Fukao T, et al. J Inherit Metab Dis. 2014 Jul;37(4):541-51. doi: 10.1007/s10545-014-9704-9. Epub 2014 Apr 8. J Inherit Metab Dis. 2014. PMID: 24706027 Review.
Cited by
- Systematic Review and Clinical Insights: The Role of the Ketogenic Diet in Managing Glioblastoma in Cancer Neuroscience.
Valerio J, Borro M, Proietti E, Pisciotta L, Olarinde IO, Fernandez Gomez M, Alvarez Pinzon AM. Valerio J, et al. J Pers Med. 2024 Aug 31;14(9):929. doi: 10.3390/jpm14090929. J Pers Med. 2024. PMID: 39338183 Free PMC article. Review. - The Key Role of the WNT/β-Catenin Pathway in Metabolic Reprogramming in Cancers under Normoxic Conditions.
Vallée A, Lecarpentier Y, Vallée JN. Vallée A, et al. Cancers (Basel). 2021 Nov 5;13(21):5557. doi: 10.3390/cancers13215557. Cancers (Basel). 2021. PMID: 34771718 Free PMC article. Review. - Investigating the Ketogenic Diet As Treatment for Primary Aggressive Brain Cancer: Challenges and Lessons Learned.
Schwartz KA, Noel M, Nikolai M, Chang HT. Schwartz KA, et al. Front Nutr. 2018 Feb 23;5:11. doi: 10.3389/fnut.2018.00011. eCollection 2018. Front Nutr. 2018. PMID: 29536011 Free PMC article. - Targeting fatty acid oxidation via Acyl-CoA binding protein hinders glioblastoma invasion.
Duman C, Di Marco B, Nevedomskaya E, Ulug B, Lesche R, Christian S, Alfonso J. Duman C, et al. Cell Death Dis. 2023 Apr 29;14(4):296. doi: 10.1038/s41419-023-05813-0. Cell Death Dis. 2023. PMID: 37120445 Free PMC article. - A ketogenic diet increases transport and oxidation of ketone bodies in RG2 and 9L gliomas without affecting tumor growth.
De Feyter HM, Behar KL, Rao JU, Madden-Hennessey K, Ip KL, Hyder F, Drewes LR, Geschwind JF, de Graaf RA, Rothman DL. De Feyter HM, et al. Neuro Oncol. 2016 Aug;18(8):1079-87. doi: 10.1093/neuonc/now088. Epub 2016 May 3. Neuro Oncol. 2016. PMID: 27142056 Free PMC article.
References
- Ohgaki H, Dessen P, Jourde B, Horstmann S, Nishikawa T, Di Patre PL, Burkhard C, Schuler D, Probst-Hensch NM, Maiorka PC, Baeza N, Pisani P, Yonekawa Y, Yasargil MG, Lutolf UM, Kleihues P. Genetic pathways to glioblastoma: a population-based study. Cancer Res. 2004;64:6892–6899. doi: 10.1158/0008-5472.CAN-04-1337. - DOI - PubMed
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous