Apolipoprotein CIII overexpressing mice are predisposed to diet-induced hepatic steatosis and hepatic insulin resistance - PubMed (original) (raw)
. 2011 Nov;54(5):1650-60.
doi: 10.1002/hep.24571. Epub 2011 Aug 19.
Andreas L Birkenfeld, Francois R Jornayvaz, Michael J Jurczak, Shoichi Kanda, Violeta Popov, David W Frederick, Dongyan Zhang, Blas Guigni, Kalyani G Bharadwaj, Cheol Soo Choi, Ira J Goldberg, Jae-Hak Park, Kitt F Petersen, Varman T Samuel, Gerald I Shulman
Affiliations
- PMID: 21793029
- PMCID: PMC3205235
- DOI: 10.1002/hep.24571
Free PMC article
Apolipoprotein CIII overexpressing mice are predisposed to diet-induced hepatic steatosis and hepatic insulin resistance
Hui-Young Lee et al. Hepatology. 2011 Nov.
Free PMC article
Abstract
Nonalcoholic fatty liver disease (NAFLD) and insulin resistance have recently been found to be associated with increased plasma concentrations of apolipoprotein CIII (APOC3) in humans carrying single nucleotide polymorphisms within the insulin response element of the APOC3 gene. To examine whether increased expression of APOC3 would predispose mice to NAFLD and hepatic insulin resistance, human APOC3 overexpressing (ApoC3Tg) mice were metabolically phenotyped following either a regular chow or high-fat diet (HFD). After HFD feeding, ApoC3Tg mice had increased hepatic triglyceride accumulation, which was associated with cellular ballooning and inflammatory changes. ApoC3Tg mice also manifested severe hepatic insulin resistance assessed by a hyperinsulinemic-euglycemic clamp, which could mostly be attributed to increased hepatic diacylglycerol content, protein kinase C-ϵ activation, and decreased insulin-stimulated Akt2 activity. Increased hepatic triglyceride content in the HFD-fed ApoC3Tg mice could be attributed to a ≈ 70% increase in hepatic triglyceride uptake and ≈ 50% reduction hepatic triglyceride secretion.
Conclusion: These data demonstrate that increase plasma APOC3 concentrations predispose mice to diet-induced NAFLD and hepatic insulin resistance.
Copyright © 2011 American Association for the Study of Liver Diseases.
Figures
Fig. 1
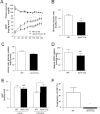
APOC3 promote diet-induced hepatic steatosis and liver damage. Animals fed an RC and HFD for 2-3 months and fasted overnight prior to taking liver and blood samples. (A) Representative histological section stained with Masson-trichrome staining, examined in ×400 light microscopy field after HFD feeding for 2-3 months (insert ×200 magnifications). Arrow indicates ballooned hepatocyte. (B) Histological scoring of NAFLD in liver section after HFD feeding for 3 months. (C) Triglyceride level in the liver of WT and ApoC3Tg mice after RC and 2-3 months of HFD feeding (n > 10). (D) Plasma ALT and AST concentration after 3 months of HFD feeding (n = 4). (E) Plasma TNF-α concentration in the WT and ApoC3Tg mice after RC and 3 months of HFD feeding (n = 3, equal amounts of 4 mice plasma were pooled into each sample). Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001 by Student's t test for (B,D) and by ANOVA with post-hoc analysis for (C,E).
Fig. 2
Hepatic steatosis in ApoC3Tg mice is associated with hepatic insulin resistance. (A) Plasma glucose concentration and glucose infusion rate in WT (n = 6) and ApoC3Tg (n = 7) mice during hyperinsulinemic-euglycemic clamp study following 2 months of HFD. (B,C) Glucose infusion rate and whole-body glucose uptake rate during steady status. (D) Insulin stimulated muscle 2-deoxy-D-[1-14C] glucose (2DOG) uptake in gastrocnemius muscle. (E) Basal EGP and insulin suppressed (clamped) EGP and (F) percentage of suppression during the hyperinsulinemic-euglycemic clamp study. Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001 by Student's t test except (A) (2-way ANOVA with post-hoc analysis).
Fig. 3
Hepatic insulin resistance in the ApoC3Tg mice is associated with increased hepatic diacylglycerol content and PKCε activation. Animals fed an RC and HFD for 2 months and fasted overnight prior to experiments. (A) Intracellular cytosolic diacylglycerol after overnight fasting. (B) Membrane translocation of PKCε was analyzed in the liver of WT and ApoC3Tg mice after hyperinsulinemic-euglycemic clamp study. (C) Akt phosphorylation on Ser473 was analyzed in the liver of overnight-fasted and hyperinsulinemic-euglycemic clamped WT and ApoC3Tg mice after 2 months of HFD feeding. Data are expressed as mean ± SEM. (A), n = 6; (B), n = 4; (C), n = 6-7 per group. *P < 0.05, **P < 0.01 by Student's t test.
Fig. 4
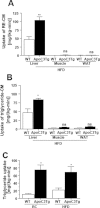
Amount of hepatic triglyceride uptake was increased in ApoC3Tg mice both on RC and HFD. Animals fed an HFD for 3 months and fasted overnight prior to experiments (A,B). After collection of blood at 0 minutes, then endogenously dual-radiolabeled chylomicron, 6 × 105 dpm of [3H]-RE-CM and 2 × 105 dpm of [14C]-triglyceride-CM were injected. Blood was collected at 2.5, 5, 10, and 15 minutes after injection (A,B). With a separate batch of animals fed RC or HFD for 3 months, after 6 hours fasting a bolus injection of 9 μCi of [3H]-triolein was intravenously administered and blood samples were collected at 2.5, 5, 7.5, 10, 15, 20, 30, and 60 minutes from tail vein (C). Tissue-specific uptake rate of (A) [3H]-RE-CM (B) [14C]-triglyceride-CM were assessed in various tissues and (C) hepatic [3H]-triolein uptake rate were assessed after RC and HFD. N = 4-5. *P < 0.05; **P < 0.01; ns, not significant by Student's t test. CM, chylomicron. RE, retinyl ester. WAT, epididymal white adipose tissue.
Fig. 5
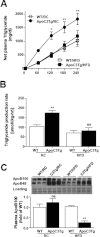
Hepatic VLDL-triglyceride secretion was decreased in ApoC3Tg mice during HFD. Animals fed an RC and HFD for 3 months and fasted overnight prior to experiments. (A) Net increase of plasma triglyceride concentration after poloxamer407 (p-407) injection from basal (0 minutes, before p-407 injection) (n = 4-6). (B) Triglyceride production rate during 4 hours after p-407 injection, expressed as micromoles of triglyceride produced per hour per kg of body weight (n = 4-6). (C) Plasma apoB determined by western blotting using 4%-12% gradient gel. The same membrane stained with Coomassie-blue was provided as loading control (n = 3, equal amount of 4 mice plasma were pooled into each sample). Data are expressed as mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001 by two-way ANOVA post-hoc analysis (A) and **P < 0.01 by two-tailed t test (B,C). ns, not significant.
Fig. 6
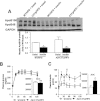
Insulin suppressed hepatic apoB100 expression and postprandial insulin secretion was increased in ApoC3Tg mice after HFD feeding. (A) Hepatic apoB100 protein expression in the liver of WT and ApoC3Tg mice from overnight fasted basal and after hyperinsulinemic-euglycemic clamp study following 2 months of HFD feeding (n = 3 for RC, n = 5 for HFD group). (B) Plasma glucose concentration and area under curve of glucose, (C) plasma insulin level and area under curve of insulin after 1 mg/kg of glucose challenge during IPGTT in WT, and ApoC3Tg mice after 6 weeks of HFD feeding (n = 7). Data are expressed as mean ± SEM. †P < 0.05 by two-way ANOVA analysis. *P < 0.05 and **P < 0.01 by two-tailed t test. ns, not significant.
Similar articles
- APOC3 Protein Is Not a Predisposing Factor for Fat-induced Nonalcoholic Fatty Liver Disease in Mice.
Cheng X, Yamauchi J, Lee S, Zhang T, Gong Z, Muzumdar R, Qu S, Dong HH. Cheng X, et al. J Biol Chem. 2017 Mar 3;292(9):3692-3705. doi: 10.1074/jbc.M116.765917. Epub 2017 Jan 23. J Biol Chem. 2017. PMID: 28115523 Free PMC article. - Apolipoprotein C3 gene variants in nonalcoholic fatty liver disease.
Petersen KF, Dufour S, Hariri A, Nelson-Williams C, Foo JN, Zhang XM, Dziura J, Lifton RP, Shulman GI. Petersen KF, et al. N Engl J Med. 2010 Mar 25;362(12):1082-9. doi: 10.1056/NEJMoa0907295. N Engl J Med. 2010. PMID: 20335584 Free PMC article. - Visceral obesity modulates the impact of apolipoprotein C3 gene variants on liver fat content.
Peter A, Kantartzis K, Machicao F, Machann J, Wagner S, Templin S, Königsrainer I, Königsrainer A, Schick F, Fritsche A, Häring HU, Stefan N. Peter A, et al. Int J Obes (Lond). 2012 Jun;36(6):774-82. doi: 10.1038/ijo.2011.154. Epub 2011 Aug 9. Int J Obes (Lond). 2012. PMID: 21829161 - [The role of apolipoprotein C3 in the regulation of nonalcoholic fatty liver disease, glucose and lipid metabolism, and islet β cell function].
Yan S, Ding ZY, Gao Y, Mao WJ, Cheng XY. Yan S, et al. Sheng Li Xue Bao. 2023 Dec 25;75(6):767-778. Sheng Li Xue Bao. 2023. PMID: 38151342 Review. Chinese. - Diacylglycerol activation of protein kinase Cε and hepatic insulin resistance.
Jornayvaz FR, Shulman GI. Jornayvaz FR, et al. Cell Metab. 2012 May 2;15(5):574-84. doi: 10.1016/j.cmet.2012.03.005. Cell Metab. 2012. PMID: 22560210 Free PMC article. Review.
Cited by
- Deletion of Jazf1 gene causes early growth retardation and insulin resistance in mice.
Lee HY, Jang HR, Li H, Samuel VT, Dudek KD, Osipovich AB, Magnuson MA, Sklar J, Shulman GI. Lee HY, et al. Proc Natl Acad Sci U S A. 2022 Dec 6;119(49):e2213628119. doi: 10.1073/pnas.2213628119. Epub 2022 Nov 28. Proc Natl Acad Sci U S A. 2022. PMID: 36442127 Free PMC article. - Lipopolysaccharide binding protein resists hepatic oxidative stress by regulating lipid droplet homeostasis.
Zhang Q, Shen X, Yuan X, Huang J, Zhu Y, Zhu T, Zhang T, Wu H, Wu Q, Fan Y, Ni J, Meng L, He A, Shi C, Li H, Hu Q, Wang J, Chang C, Huang F, Li F, Chen M, Liu A, Ye S, Zheng M, Fang H. Zhang Q, et al. Nat Commun. 2024 Apr 13;15(1):3213. doi: 10.1038/s41467-024-47553-5. Nat Commun. 2024. PMID: 38615060 Free PMC article. - Deletion of the diabetes candidate gene Slc16a13 in mice attenuates diet-induced ectopic lipid accumulation and insulin resistance.
Schumann T, König J, von Loeffelholz C, Vatner DF, Zhang D, Perry RJ, Bernier M, Chami J, Henke C, Kurzbach A, El-Agroudy NN, Willmes DM, Pesta D, de Cabo R, O Sullivan JF, Simon E, Shulman GI, Hamilton BS, Birkenfeld AL. Schumann T, et al. Commun Biol. 2021 Jul 1;4(1):826. doi: 10.1038/s42003-021-02279-8. Commun Biol. 2021. PMID: 34211098 Free PMC article. - Apolipoprotein C-III and its defined lipoprotein subspecies in relation to incident diabetes: the Multi-Ethnic Study of Atherosclerosis.
Aroner SA, Furtado JD, Sacks FM, Tsai MY, Mukamal KJ, McClelland RL, Jensen MK. Aroner SA, et al. Diabetologia. 2019 Jun;62(6):981-992. doi: 10.1007/s00125-019-4847-8. Epub 2019 Apr 4. Diabetologia. 2019. PMID: 30949716 - Pathophysiology of NASH in endocrine diseases.
Gariani K, Jornayvaz FR. Gariani K, et al. Endocr Connect. 2021 Feb;10(2):R52-R65. doi: 10.1530/EC-20-0490. Endocr Connect. 2021. PMID: 33449917 Free PMC article. Review.
References
- Seppala-Lindroos A, Vehkavaara S, Hakkinen AM, Goto T, Westerbacka J, Sovijarvi A, et al. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab. 2002;87:3023–3028. - PubMed
- Ito Y, Azrolan N, O'Connell A, Walsh A, Breslow JL. Hypertriglyceridemia as a result of human apo CIII gene expression in transgenic mice. Science. 1990;249:790–793. - PubMed
MeSH terms
Substances
Grants and funding
- R01 DK049230/DK/NIDDK NIH HHS/United States
- U24 DK059635/DK/NIDDK NIH HHS/United States
- R01 DK040936/DK/NIDDK NIH HHS/United States
- R24 DK085638/DK/NIDDK NIH HHS/United States
- R01 AG023686/AG/NIA NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous