Thailandepsins: bacterial products with potent histone deacetylase inhibitory activities and broad-spectrum antiproliferative activities - PubMed (original) (raw)
. 2011 Oct 28;74(10):2031-8.
doi: 10.1021/np200324x. Epub 2011 Jul 27.
Affiliations
- PMID: 21793558
- PMCID: PMC3204160
- DOI: 10.1021/np200324x
Thailandepsins: bacterial products with potent histone deacetylase inhibitory activities and broad-spectrum antiproliferative activities
Cheng Wang et al. J Nat Prod. 2011.
Abstract
Histone deacetylase (HDAC) inhibitors have emerged as a new class of anticancer drugs, with one synthetic compound, SAHA (vorinostat, Zolinza; 1), and one natural product, FK228 (depsipeptide, romidepsin, Istodax; 2), approved by FDA for clinical use. Our studies of FK228 biosynthesis in Chromobacterium violaceum no. 968 led to the identification of a cryptic biosynthetic gene cluster in the genome of Burkholderia thailandensis E264. Genome mining and genetic manipulation of this gene cluster further led to the discovery of two new products, thailandepsin A (6) and thailandepsin B (7). HDAC inhibition assays showed that thailandepsins have selective inhibition profiles different from that of FK228, with comparable inhibitory activities to those of FK228 toward human HDAC1, HDAC2, HDAC3, HDAC6, HDAC7, and HDAC9 but weaker inhibitory activities than FK228 toward HDAC4 and HDAC8, the latter of which could be beneficial. NCI-60 anticancer screening assays showed that thailandepsins possess broad-spectrum antiproliferative activities with GI50 for over 90% of the tested cell lines at low nanomolar concentrations and potent cytotoxic activities toward certain types of cell lines, particularly for those derived from colon, melanoma, ovarian, and renal cancers. Thailandepsins thus represent new naturally produced HDAC inhibitors that are promising for anticancer drug development.
Figures
Figure 1. Comparison of two homologous biosynthetic gene clusters and discrete genes necessary for the biosynthesis of FK228 (2) or thailandepsins A (6) and B (7)
Each gene cluster is depicted in a row, under which is the deduced respective modular biosynthetic pathway. A, ACP, AL, AT, C, DH, E, KR, KS, PCP and TE are standard abbreviations of the nonribosomal peptide synthetase (NRPS) or polyketide synthase (PKS) domain names whose full name and respective function can be found in Fischbach et al. Sfp and AT are the generic protein names of their respective genes defined in the main text.
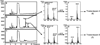
Figure 2. Metabolic profiling of the wild type strain (BthWT) and BthΔ_tdpAB_ mutant strain of B. thailandensis E264
Panels a, b and c are the HPLC traces of extract of the medium control, BthWT strain or BthΔ_tdpAB_ strain, respectively. Panels d and e are ESI-LC-MS spectra of the HPLC Peak 1 and Peak 2 of the BthWT sample (Panel b). Panels f and g are ESI-LC-MS spectra of the reduced HPLC Peak 1 and Peak 2 of the BthWT sample (Panel b), showing a +2 m/z shift of every ion signal.
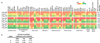
Figure 3. In vitro antiproliferative/cytotoxic activities of thailandepsins A (6) and B (7) in comparison with FK228 (2)
(a) Heat map representation of antiproliferative/cytotoxic activities of 6 and 7 across NCI-60 cell lines, with higher activity (lower index value) in warmer (darker if printed in black-white) color. GI50, growth-inhibition indicator; TGI, cytostatic effect indicator; LC50, cytotoxic effect indicator. Compound concentration in M was converted to log10 value for continuous color scale transformation. Mean panel response values were expressed in µM. (b) Statistics comparison of antiproliferation/cytotoxicity profile between 2, 6, and 7 across NCI-60 cell lines.
Similar articles
- Histone deacetylase inhibitors from Burkholderia thailandensis.
Klausmeyer P, Shipley SM, Zuck KM, McCloud TG. Klausmeyer P, et al. J Nat Prod. 2011 Oct 28;74(10):2039-44. doi: 10.1021/np200532d. Epub 2011 Oct 3. J Nat Prod. 2011. PMID: 21967146 Free PMC article. - Tropolones and Thailandepsin B as Lead-like Natural Compounds in the Development of Potent and Selective Histone Deacetylase Inhibitors.
Pal D, Lal P. Pal D, et al. Curr Drug Targets. 2023;24(9):698-717. doi: 10.2174/1389450124666230707144251. Curr Drug Targets. 2023. PMID: 37424350 - Acyldepsipeptide HDAC inhibitor production induced in Burkholderia thailandensis.
Biggins JB, Gleber CD, Brady SF. Biggins JB, et al. Org Lett. 2011 Mar 18;13(6):1536-9. doi: 10.1021/ol200225v. Epub 2011 Feb 24. Org Lett. 2011. PMID: 21348454 Free PMC article. - Romidepsin (FK228), A Histone Deacetylase Inhibitor and its Analogues in Cancer Chemotherapy.
Pojani E, Barlocco D. Pojani E, et al. Curr Med Chem. 2021;28(7):1290-1303. doi: 10.2174/0929867327666200203113926. Curr Med Chem. 2021. PMID: 32013816 Review. - Histone deacetylase inhibitors from microorganisms: the Astellas experience.
Masuoka Y, Shindoh N, Inamura N. Masuoka Y, et al. Prog Drug Res. 2008;66:335, 337-59. doi: 10.1007/978-3-7643-8595-8_7. Prog Drug Res. 2008. PMID: 18416310 Review.
Cited by
- Computational design of a time-dependent histone deacetylase 2 selective inhibitor.
Zhou J, Li M, Chen N, Wang S, Luo HB, Zhang Y, Wu R. Zhou J, et al. ACS Chem Biol. 2015 Mar 20;10(3):687-92. doi: 10.1021/cb500767c. Epub 2015 Jan 2. ACS Chem Biol. 2015. PMID: 25546141 Free PMC article. - Antineoplastic effects of histone deacetylase inhibitors in neuroendocrine cancer cells are mediated through transcriptional regulation of Notch1 by activator protein 1.
Jang S, Jin H, Roy M, Ma AL, Gong S, Jaskula-Sztul R, Chen H. Jang S, et al. Cancer Med. 2017 Sep;6(9):2142-2152. doi: 10.1002/cam4.1151. Epub 2017 Aug 4. Cancer Med. 2017. PMID: 28776955 Free PMC article. - Target engagement and drug residence time can be observed in living cells with BRET.
Robers MB, Dart ML, Woodroofe CC, Zimprich CA, Kirkland TA, Machleidt T, Kupcho KR, Levin S, Hartnett JR, Zimmerman K, Niles AL, Ohana RF, Daniels DL, Slater M, Wood MG, Cong M, Cheng YQ, Wood KV. Robers MB, et al. Nat Commun. 2015 Dec 3;6:10091. doi: 10.1038/ncomms10091. Nat Commun. 2015. PMID: 26631872 Free PMC article. - Overexpression of somatostatin receptor type 2 in neuroendocrine tumors for improved Ga68-DOTATATE imaging and treatment.
Guenter R, Aweda T, Carmona Matos DM, Jang S, Whitt J, Cheng YQ, Liu XM, Chen H, Lapi SE, Jaskula-Sztul R. Guenter R, et al. Surgery. 2020 Jan;167(1):189-196. doi: 10.1016/j.surg.2019.05.092. Epub 2019 Oct 16. Surgery. 2020. PMID: 31629542 Free PMC article. - Inhibition of HDAC1 and DNMT1 modulate RGS10 expression and decrease ovarian cancer chemoresistance.
Cacan E, Ali MW, Boyd NH, Hooks SB, Greer SF. Cacan E, et al. PLoS One. 2014 Jan 27;9(1):e87455. doi: 10.1371/journal.pone.0087455. eCollection 2014. PLoS One. 2014. PMID: 24475290 Free PMC article.
References
- Yoo CB, Jones PA. Nat. Rev. Drug Discov. 2006;5:37–50. - PubMed
- Bolden JE, Peart MJ, Johnstone RW. Nat. Rev. Drug Discov. 2006;5:769–784. - PubMed
- Bots M, Johnstone RW. Clin. Cancer Res. 2009;15:3970–3977. - PubMed
- Lane AA, Chabner BA. J. Clin. Oncol. 2009;27:5459–5468. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Miscellaneous