Chloroplast lipid droplet type II NAD(P)H quinone oxidoreductase is essential for prenylquinone metabolism and vitamin K1 accumulation - PubMed (original) (raw)
Chloroplast lipid droplet type II NAD(P)H quinone oxidoreductase is essential for prenylquinone metabolism and vitamin K1 accumulation
Lucia Eugeni Piller et al. Proc Natl Acad Sci U S A. 2011.
Abstract
Lipid droplets are ubiquitous cellular structures in eukaryotes and are required for lipid metabolism. Little is currently known about plant lipid droplets other than oil bodies. Here, we define dual roles for chloroplast lipid droplets (plastoglobules) in energy and prenylquinone metabolism. The prenylquinones--plastoquinone, plastochromanol-8, phylloquinone (vitamin K(1)), and tocopherol (vitamin E)--are partly stored in plastoglobules. This work shows that NAD(P)H dehydrogenase C1 (NDC1) (At5g08740), a type II NAD(P)H quinone oxidoreductase, associates with plastoglobules. NDC1 reduces a plastoquinone analog in vitro and affects the overall redox state of the total plastoquinone pool in vivo by reducing the plastoquinone reservoir of plastoglobules. Finally, NDC1 is required for normal plastochromanol-8 accumulation and is essential for vitamin K(1) production.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
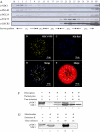
NDC1 lipid droplet localization. (A) Western blotting of chloroplast membrane fractions separated by sucrose-gradient flotation. Toc75 and LHCB2 are envelope and thylakoid markers, respectively. PGL35 and PGL40 are plastoglobule markers. CP, total chloroplast; St, stroma. (B) NDC1-YFP under the control of the cauliflower mosaic virus 35S promoter was transiently expressed in tobacco protoplasts. Transformed protoplasts were analyzed by confocal laser microscopy. (C) The neutral lipid dye Nile Red reveals lipid droplets in chloroplasts (plastoglobules). (D) The first merged image shows the superposition of NDC1-YFP and Nile Red in white. (E) The second merged image shows the chlorophyll autofluorescence to visualize chloroplasts. (F) 35S-labeled pre-NDC1 (lane 1) was incubated with isolated chloroplasts in vitro in a time-course experiment (0, 2, 5, and 10 min, lanes 2–5). The imported, lower molecular mass NDC1 was resistant to exogenously added thermolysin protease (lane 6). (G) 35S-labeled pre-NDC1 was incubated with isolated mitochondria in vitro (lane 7). The experiment was analyzed at the 0- and 30-min time points (lanes 8 and 10). The imported, lower molecular mass NDC1 was resistant to exogenously added proteinase K (lanes 9 and 11).
Fig. 2.
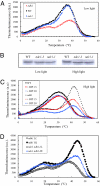
Thermoluminescence measurements in WT, ndc1, and ndh1 mutant leaves of Arabidopsis. (A) Far-red light-induced thermoluminescence signal in WT Arabidopsis leaves and in mutant leaves (ndc1, ndh1) grown under standard conditions. The band peaking at temperature >35 °C is the so-called afterglow band. The shoulder observed at lower temperature (∼20 °C) is a B band corresponding to the S2/3 QB− charge recombination. The data are representative traces of at least six separate experiments. (B) Immunodetection of NDC1 in total extracts of WT and two ndc1 mutant lines (ndc1-1 and ndc1-3) under normal and high-light growth conditions. The upper band, weakly visible in the WT under low-light conditions and more strongly under high-light conditions, corresponds to the NDC1 protein. The band is absent from the ndc1-1 and ndc1-3 mutants. The lower band is a nonspecific, cross-reacting signal. (C) Effects of DPI (15 and 30 μM) on the afterglow thermoluminescence band of WT and ndh1 mutant leaves acclimated for 7 d to high light. Data are representative traces of three separate experiments; (D) Afterglow thermoluminescence band measured in leaves of the ndh1 mutant and the ndc1 ndh1 mutant acclimated for 7 d to high light. Data are representative traces of at least six separate experiments.
Fig. 3.
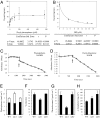
NDC1 enzyme activity and prenylquinone quantification in the ndc1 and ndh1 mutants. (A) NDC1 activity was measured by monitoring NADPH oxidation in the presence of increasing concentrations of decyl-PQ (a PQ analog). (B) Increasing concentrations of the inhibitor DPI were added to the assay in the presence of 100 μM decyl-PQ. The kinetic parameters were calculated with SigmaPlot software. (A and B) Each data point represents two (B) and three (A) experimental replicates. (C and D) NDC1 activity was measured in the presence of isolated plastoglobules (C) and in the presence of decyl-ubiquinone and NADH (D). The arrows indicate the time point of addition of purified NDC1. (E–H) Prenylquinones in leaves determined by HPLC. (E) Total PQ. The white bar indicates the fraction of oxidized PQ. (F) Percentage reduction of the PQ pool. (G) PC-8. (H) α-Tocopherol in WT Arabidopsis leaves and ndc1 or ndh1 mutant leaves grown under standard conditions. Data are mean values of at least three separate experiments.
Fig. 4.
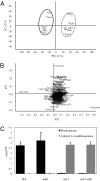
Untargeted lipidomics of ndc1 mutants. (A) Methanolic extracts from leaves of ndc1, ndh1, and ndc1 ndh1 (dk) mutants lines were analyzed with UHPLC-QTOFMS. (A) Principal component analysis score plots (PC1 × PC2) with their percentages of explained variance, based on normalized data from negative ion UHPLC-QTOFMS analyses. (B) Corresponding loading plot showing the most relevant variables responsible for the separation found in the score plot (436.3336 on far left corresponds to 2-phytyl-1,4-naphthoquinone; 450.3494 on far right corresponds to phylloquinone). (C) Quantification of phylloquinone and 2-phytyl-1,4-naphthoquinone in WT (Col0) and ndc1, ndh1, and ndc1 ndh1 mutant plants obtained from a standard solution of phylloquinone. Data represent the mean of two independent experiments (n = 3 each).
Similar articles
- A chloroplast ABC1-like kinase regulates vitamin E metabolism in Arabidopsis.
Martinis J, Glauser G, Valimareanu S, Kessler F. Martinis J, et al. Plant Physiol. 2013 Jun;162(2):652-62. doi: 10.1104/pp.113.218644. Epub 2013 Apr 30. Plant Physiol. 2013. PMID: 23632854 Free PMC article. - Functional modeling identifies paralogous solanesyl-diphosphate synthases that assemble the side chain of plastoquinone-9 in plastids.
Block A, Fristedt R, Rogers S, Kumar J, Barnes B, Barnes J, Elowsky CG, Wamboldt Y, Mackenzie SA, Redding K, Merchant SS, Basset GJ. Block A, et al. J Biol Chem. 2013 Sep 20;288(38):27594-27606. doi: 10.1074/jbc.M113.492769. Epub 2013 Aug 2. J Biol Chem. 2013. PMID: 23913686 Free PMC article. - Prenylquinone profiling in whole leaves and chloroplast subfractions.
Kessler F, Glauser G. Kessler F, et al. Methods Mol Biol. 2014;1153:213-26. doi: 10.1007/978-1-4939-0606-2_15. Methods Mol Biol. 2014. PMID: 24777800 - Plastid lipid droplets at the crossroads of prenylquinone metabolism.
Eugeni Piller L, Abraham M, Dörmann P, Kessler F, Besagni C. Eugeni Piller L, et al. J Exp Bot. 2012 Feb;63(4):1609-18. doi: 10.1093/jxb/ers016. J Exp Bot. 2012. PMID: 22371323 Review. - Plastochromanol-8: fifty years of research.
Kruk J, Szymańska R, Cela J, Munne-Bosch S. Kruk J, et al. Phytochemistry. 2014 Dec;108:9-16. doi: 10.1016/j.phytochem.2014.09.011. Epub 2014 Oct 9. Phytochemistry. 2014. PMID: 25308762 Review.
Cited by
- A mechanism implicating plastoglobules in thylakoid disassembly during senescence and nitrogen starvation.
Besagni C, Kessler F. Besagni C, et al. Planta. 2013 Feb;237(2):463-70. doi: 10.1007/s00425-012-1813-9. Epub 2012 Nov 28. Planta. 2013. PMID: 23187680 Review. - The evolution of strictly monofunctional naphthoquinol C-methyltransferases is vital in cyanobacteria and plastids.
Stutts L, Latimer S, Batyrshina Z, Dickinson G, Alborn H, Block AK, Basset GJ. Stutts L, et al. Plant Cell. 2023 Sep 27;35(10):3686-3696. doi: 10.1093/plcell/koad202. Plant Cell. 2023. PMID: 37477936 Free PMC article. - A systematic in silico report on iron and zinc proteome of Zea mays.
Sharma A, Sharma D, Verma SK. Sharma A, et al. Front Plant Sci. 2023 Aug 17;14:1166720. doi: 10.3389/fpls.2023.1166720. eCollection 2023. Front Plant Sci. 2023. PMID: 37662157 Free PMC article. - Altering the balance between AOX1A and NDB2 expression affects a common set of transcripts in Arabidopsis.
Sweetman C, Waterman CD, Wong DCJ, Day DA, Jenkins CLD, Soole KL. Sweetman C, et al. Front Plant Sci. 2022 Nov 15;13:876843. doi: 10.3389/fpls.2022.876843. eCollection 2022. Front Plant Sci. 2022. PMID: 36466234 Free PMC article. - Chromoplast plastoglobules recruit the carotenoid biosynthetic pathway and contribute to carotenoid accumulation during tomato fruit maturation.
Zita W, Bressoud S, Glauser G, Kessler F, Shanmugabalaji V. Zita W, et al. PLoS One. 2022 Dec 6;17(12):e0277774. doi: 10.1371/journal.pone.0277774. eCollection 2022. PLoS One. 2022. PMID: 36472971 Free PMC article.
References
- Shikanai T. Cyclic electron transport around photosystem I: Genetic approaches. Annu Rev Plant Biol. 2007;58:199–217. - PubMed
- Rumeau D, Peltier G, Cournac L. Chlororespiration and cyclic electron flow around PSI during photosynthesis and plant stress response. Plant Cell Environ. 2007;30:1041–1051. - PubMed
- Johnson GN. Physiology of PSI cyclic electron transport in higher plants. Biochim Biophys Acta. 2011;1807:384–389. - PubMed
- Cardol P, Forti G, Finazzi G. Regulation of electron transport in microalgae. Biochim Biophys Acta. 2011;1807:912–918. - PubMed
- Munekage Y, et al. PGR5 is involved in cyclic electron flow around photosystem I and is essential for photoprotection in Arabidopsis. Cell. 2002;110:361–371. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases