All clinically-relevant blood components transmit prion disease following a single blood transfusion: a sheep model of vCJD - PubMed (original) (raw)
doi: 10.1371/journal.pone.0023169. Epub 2011 Aug 17.
Anthony Richard Alejo Blanco, E Fiona Houston, Christopher de Wolf, Boon Chin Tan, Antony Smith, Martin H Groschup, Nora Hunter, Valerie S Hornsey, Ian R MacGregor, Christopher V Prowse, Marc Turner, Jean C Manson
Affiliations
- PMID: 21858015
- PMCID: PMC3157369
- DOI: 10.1371/journal.pone.0023169
All clinically-relevant blood components transmit prion disease following a single blood transfusion: a sheep model of vCJD
Sandra McCutcheon et al. PLoS One. 2011.
Abstract
Variant CJD (vCJD) is an incurable, infectious human disease, likely arising from the consumption of BSE-contaminated meat products. Whilst the epidemic appears to be waning, there is much concern that vCJD infection may be perpetuated in humans by the transfusion of contaminated blood products. Since 2004, several cases of transfusion-associated vCJD transmission have been reported and linked to blood collected from pre-clinically affected donors. Using an animal model in which the disease manifested resembles that of humans affected with vCJD, we examined which blood components used in human medicine are likely to pose the greatest risk of transmitting vCJD via transfusion. We collected two full units of blood from BSE-infected donor animals during the pre-clinical phase of infection. Using methods employed by transfusion services we prepared red cell concentrates, plasma and platelets units (including leucoreduced equivalents). Following transfusion, we showed that all components contain sufficient levels of infectivity to cause disease following only a single transfusion and also that leucoreduction did not prevent disease transmission. These data suggest that all blood components are vectors for prion disease transmission, and highlight the importance of multiple control measures to minimise the risk of human to human transmission of vCJD by blood transfusion.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
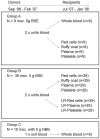
Figure 1. Overview of experimental design.
Sheep used as BSE blood donors were divided into two experimental groups (A and B), though both groups were orally inoculated (inoc.) with 5 g of BSE brain homogenate at approximately 3–6 months of age. Two units of blood were collected from all BSE donors and processed according to the experimental regime described: group A sheep - one unit was transfused to recipients as whole blood, whereas blood from the second unit was processed into components and then transfused. Both units of blood from sheep in group B were separated into components and one set of components was passed through human leucoreduction (LR) filters prior to transfusion. Negative control donors (group C), were orally inoculated with normal bovine brain homogenate (NBB). A unit of whole blood was collected from these sheep and transfused to recipients.
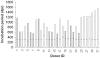
Figure 2. Varied incubation times are seen in blood donors following oral inoculation with BSE.
All BSE-infected donor sheep (apart from donor 4, which was culled for welfare reasons) were culled on demonstration of clinical signs. The range of incubation period for sheep confirmed as having BSE is 534–1544 days post infection (dpi). The horizontal bar in each column represents the time of blood collection for separation and subsequent transfusion to recipients and ranges from 228–463 days post oral inoculation. To date, 31/39 donors have succumbed to BSE infection and the grey bars indicate donor blood, which when transfused, caused disease in recipients.
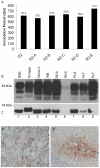
Figure 3. Analysis of sheep blood components.
Graph A shows the range and average volume (±1SD) of each component used for transfusion. No difference was observed in the volume between leucoreduced (grey bars) and non-leucoreduced components (black bars). White cell counts were calculated using Leucocount™ kits and flow cytometry. All leucoreduced components from sheep (grey bars) meet the specifications assigned to human equivalents and contained less that 1×106 residual leucocytes (as shown by the solid line, graph B). Following the separation of whole blood, the majority of the total plasma volume was distributed in association with plasma unit. The remaining plasma volume was differentially distributed in red cells, platelets and buffy coat, (panel C) as a percentage of the total plasma volume. No major differences were observed in plasma distribution between leucodepleted and non-leucodepleted components. The number of platelets in platelet concentrates prepared from leucoreduced and non-leucoreduced components was highly variable in sheep and the average count was statistically significantly different (p<1×10−5, using 2-tailed Student's t-test, graph D).
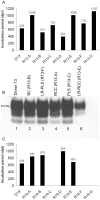
Figure 4. Confirmation of BSE in recipients of non-leucoreduced components.
Panel A shows the incubation periods of donor 2 (D2) and its respective transfusion recipients of whole blood (R2-A), red cells (R2-B), buffy coat (R2-C), plasma (R2-D) and platelets (R2-E), whereby the nomenclature used to describe sheep is consistent with that in Table 1. Panel B shows PrPSc in brain from a donor and a range of transfusion recipients compared to PrPSc in experimental controls. Lane 1 – BSE control, lane 2 – sheep scrapie control, lane 3 – donor 2, lane 4 whole blood recipient, lanes 5 and 6 – two recipients of red cells, lane 7 – plasma recipient and lanes 8 & 9 – two recipients of platelets. Lane 6 shows detection of low levels of PrPSc in recipient 7B that received a unit of red cell concentrate and died of intercurrent causes before reaching clinical endpoints. The PrPSc profile is consistent with that of cattle BSE (lane 1) as expected. These data are confirmed following PNGase F treatment of PrPSc as shown in panel C. Panels D and E show abnormal prion protein deposition in dorsal motor nuclei of the vagus nerve (DMNV) in brain and tonsil of donor 16, respectively. Tissue sections were stained with BG4.
Figure 5. BSE is transmitted following the transfusion of leucoreduced components.
Panel A shows the entire repertoire of components prepared from donor 13 (D13) and transfused. The corresponding incubation periods (defined as the date of death based on reaching defined clinical endpoints) is shown in days above the bars. Both platelet recipients (R13-D and R13-G) did not reach clinical endpoint, but were culled for welfare reasons (thus these ‘incubation periods’ correctly refer to the date of euthanasia, in days post transfusion (dpt)). Panel B is a representative immunoblot showing PrPSc in brain samples of donor 13 (lane 1) and selected, matched transfusion recipients of buffy coat, leucoreduced plasma, red cells, plasma and leucoreduced red cells (lanes 2–6 respectively), thus confirms BSE infection following the transfusion of leucoreduced blood components. Selected recipients of components, including leucoreduced red cells, from donor 19 were also confirmed to have BSE. All of these recipients developed a clinical disease at different time points post transfusion (panel C). Blood from donors 13 and 19 was collected at 48 and 52% of the final incubation period respectively; all later showed typical clinical signs of BSE and were confirmed as positive, as previously described.
Comment in
- Leucoreduction of blood components: an effective way to increase blood safety?
Bianchi M, Vaglio S, Pupella S, Marano G, Facco G, Liumbruno GM, Grazzini G. Bianchi M, et al. Blood Transfus. 2016 May;14(2):214-27. doi: 10.2450/2015.0154-15. Epub 2015 Dec 16. Blood Transfus. 2016. PMID: 26710353 Free PMC article. Review.
Similar articles
- Can prion diseases be transmitted between individuals via blood transfusion: evidence from sheep experiments.
Hunter N, Houston F. Hunter N, et al. Dev Biol (Basel). 2002;108:93-8. Dev Biol (Basel). 2002. PMID: 12220147 - Transmission of BSE by blood transfusion in sheep.
Houston F, Foster JD, Chong A, Hunter N, Bostock CJ. Houston F, et al. Lancet. 2000 Sep 16;356(9234):999-1000. doi: 10.1016/s0140-6736(00)02719-7. Lancet. 2000. PMID: 11041403 - Preclinical transmission of prions by blood transfusion is influenced by donor genotype and route of infection.
Salamat MKF, Blanco ARA, McCutcheon S, Tan KBC, Stewart P, Brown H, Smith A, de Wolf C, Groschup MH, Becher D, Andréoletti O, Turner M, Manson JC, Houston EF. Salamat MKF, et al. PLoS Pathog. 2021 Feb 18;17(2):e1009276. doi: 10.1371/journal.ppat.1009276. eCollection 2021 Feb. PLoS Pathog. 2021. PMID: 33600501 Free PMC article. - Risks of transmission of variant Creutzfeldt-Jakob disease by blood transfusion.
Peden AH, Ritchie DL, Ironside JW. Peden AH, et al. Folia Neuropathol. 2005;43(4):271-8. Folia Neuropathol. 2005. PMID: 16416391 Review. - [BSE, prions, vCJD and (not only) homologous transfusion].
Köhler M. Köhler M. Anasthesiol Intensivmed Notfallmed Schmerzther. 2003 Jan;38(1):43-7. doi: 10.1055/s-2003-36564. Anasthesiol Intensivmed Notfallmed Schmerzther. 2003. PMID: 12522730 Review. German. No abstract available.
Cited by
- Highly efficient prion transmission by blood transfusion.
Andréoletti O, Litaise C, Simmons H, Corbière F, Lugan S, Costes P, Schelcher F, Vilette D, Grassi J, Lacroux C. Andréoletti O, et al. PLoS Pathog. 2012;8(6):e1002782. doi: 10.1371/journal.ppat.1002782. Epub 2012 Jun 21. PLoS Pathog. 2012. PMID: 22737075 Free PMC article. - In vitro detection of prionemia in TSE-infected cervids and hamsters.
Elder AM, Henderson DM, Nalls AV, Wilham JM, Caughey BW, Hoover EA, Kincaid AE, Bartz JC, Mathiason CK. Elder AM, et al. PLoS One. 2013 Nov 1;8(11):e80203. doi: 10.1371/journal.pone.0080203. eCollection 2013. PLoS One. 2013. PMID: 24224043 Free PMC article. - Variant CJD. 18 years of research and surveillance.
Diack AB, Head MW, McCutcheon S, Boyle A, Knight R, Ironside JW, Manson JC, Will RG. Diack AB, et al. Prion. 2014;8(4):286-95. doi: 10.4161/pri.29237. Epub 2014 Nov 1. Prion. 2014. PMID: 25495404 Free PMC article. Review. - Human stem cell-derived astrocytes replicate human prions in a PRNP genotype-dependent manner.
Krejciova Z, Alibhai J, Zhao C, Krencik R, Rzechorzek NM, Ullian EM, Manson J, Ironside JW, Head MW, Chandran S. Krejciova Z, et al. J Exp Med. 2017 Dec 4;214(12):3481-3495. doi: 10.1084/jem.20161547. Epub 2017 Nov 15. J Exp Med. 2017. PMID: 29141869 Free PMC article. - Implications of peptide assemblies in amyloid diseases.
Ke PC, Sani MA, Ding F, Kakinen A, Javed I, Separovic F, Davis TP, Mezzenga R. Ke PC, et al. Chem Soc Rev. 2017 Oct 30;46(21):6492-6531. doi: 10.1039/c7cs00372b. Chem Soc Rev. 2017. PMID: 28702523 Free PMC article. Review.
References
- Will RG, Ironside JW, Zeidler M, Cousens SN, Estibeiro K, et al. A new variant of Creutzfeldt-Jakob disease in the UK. Lancet. 1996;347:921–925. - PubMed
- Bruce ME, Will RG, Ironside JW, McConnell I, Drummond D, et al. Transmissions to mice indicate that ‘new variant’ CJD is caused by the BSE agent. Nature. 1997;389:498–501. - PubMed
- Bruce ME, McConnell I, Will RG, Ironside JW. Detection of variant Creutzfeld-Jakob disease infectivity in extraneural tissues. Lancet. 2001;358:208–209. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BBS/E/D/05241339/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/D/05241340/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 007/0162/DH_/Department of Health/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical