Oncogenic YAP promotes radioresistance and genomic instability in medulloblastoma through IGF2-mediated Akt activation - PubMed (original) (raw)
Oncogenic YAP promotes radioresistance and genomic instability in medulloblastoma through IGF2-mediated Akt activation
A Fernandez-L et al. Oncogene. 2012.
Abstract
Radiation therapy remains the standard of care for many cancers, including the malignant pediatric brain tumor medulloblastoma. Radiation leads to long-term side effects, whereas radioresistance contributes to tumor recurrence. Radio-resistant medulloblastoma cells occupy the perivascular niche. They express Yes-associated protein (YAP), a Sonic hedgehog (Shh) target markedly elevated in Shh-driven medulloblastomas. Here we report that YAP accelerates tumor growth and confers radioresistance, promoting ongoing proliferation after radiation. YAP activity enables cells to enter mitosis with un-repaired DNA through driving insulin-like growth factor 2 (IGF2) expression and Akt activation, resulting in ATM/Chk2 inactivation and abrogation of cell cycle checkpoints. Our results establish a central role for YAP in counteracting radiation-based therapies and driving genomic instability, and indicate the YAP/IGF2/Akt axis as a therapeutic target in medulloblastoma.
Figures
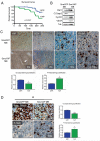
Figure 1. YAP promotes medulloblastoma cell tumorigenicity, proliferation and survival
(A) Tumor-free survival curve. Two hundred thousand NeuroD2-SmoA1 tumor cells transduced with either GFP or YAP were implanted into the brain of NOD/SCID post-natal day 2 pups. Animals were sacrificed when they developed symptoms. YAP-over-expressing medulloblastomas occurred with a higher penetrance and earlier development compared to GFP-transduced tumors (Long-rank Test, p=0.0047). 14/17 animals implanted with YAP-SmoA1 medulloblastomas cells developed tumors while only 8/17 animals implanted with GFP-SmoA1 cells did. A similar trend was observed when nonimmunocompromised recipient pups were used (Long-rank Test, p=0.0487; Supplementary Figure 1). (B) Representative western blot analysis of GFP-SmoA1 and YAP-SmoA1 medulloblastomas. Note reduced cleaved caspase 3, increased cyclin D2, and increased VEGF in YAP-SmoA1 medulloblastoma. (C) Immunohistochemical analysis of YAP, the endothelial cell marker CD31, cleaved caspase 3, and phosphorylated histone H3 in GFP-SmoA1 (top) and YAP-SmoA1 (bottom) medulloblastomas. YAP-SmoA1 tumors have significantly reduced apoptosis and increased proliferation compared with GFP-SmoA1 tumors, indicated in the graphs. (D) Immunohistochemical analysis and quantification of apoptosis (cleaved caspase 3) and proliferation (phosphorylated histone H3) in GFP-SmoA1 and YAP-SmoA1 medulloblastomas three hours after tumor-bearing animals were irradiated (2 Gy). Statistically significant differences are indicated as (*) P < 0.05; (***) P < 0.001.
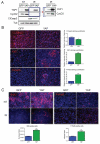
Figure 2. YAP promotes proliferation and survival in irradiated CGNPs
(A) Western blot analysis of proliferation (cyclin D2) and apoptosis (cleaved caspase 3) in GFP- or YAP-transduced Shh-treated CGNPs untreated (NT) and 20 hours after γ-irradiation (IR) with 10 Gy. Panel to the right shows over-exposed image. Similar infection efficiencies were obtained with both retroviruses (Supplementary Figure 2). (B) Immunofluorescence analysis and quantification of cleaved caspase 3 and the proliferation markers Ki67 and phosphorylated histone H3 (P-Hist3) in GFP- or YAP-transduced CGNPs 20 hours post-irradiation. (C) Left, immunofluorescence analysis of BrdU incorporation (1-hour pulse) untreated (NT; top panels) and 18 hours after irradiation (IR; middle panels). The S-phase ratios for GFP and YAP-transduced CGNPs (%BrdU-positive of treated / %BrdU-positive untreated) are shown at the bottom. Irradiated GFP-transduced cells were normalized to non-irradiated GFP-transduced CGNPs; irradiated YAP-transduced CGNPs were normalized to non-irradiated YAP-transduced CGNPs. The higher S-phase ratio indicates that YAP-over-expressing CGNPs present a defect in the G1/S checkpoint. Right, immunofluorescence analysis of phosphorylated histone H3 before (NT; top panels) and 1 hour after radiation (IR; middle panels) in GFP- or YAP-transduced CGNPs. YAP-expressing CGNPs showed a higher mitotic ratio (%P-Hist3 positive of treated / % P-Hist3 positive untreated) (bottom right), indicating failure of the G2/M checkpoint in these cells. Again, irradiated GFP-transduced cells were normalized to non-irradiated GFP-transduced CGNPs; irradiated YAP-transduced CGNPs were normalized to non-irradiated YAP-transduced CGNPs. Statistically significant differences are indicated as (*) P < 0.05; (***) P < 0.001.
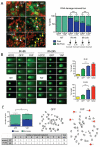
Figure 3. YAP over-expression prevents complete DNA repair
(A) Immunostaining (left) for 53BP1, which marks sites of DNA damage (“foci”) in control (NT) or irradiated (IR) CGNPs infected with either GFP or YAP. Quantification shown at right indicates that fewer YAP-transduced cells contained foci compared to GFP-transduced CGNPs, and YAP-transduced cells contained fewer foci/cell. (B) Comet assay to detect DNA damage performed in CGNPs 9 and 24 hours post-irradiation. H2O2 serves as a positive control for detection of damaged DNA by this assay. YAP-over-expressing cells contained more damaged DNA at both time points, as measured by the length of the comets’ tails (quantification at right). (C) (Left) Quantification of chromatid and chromosome breaks in metaphase spreads from GFP- or YAP-transduced CGNPs twenty-four hours after irradiation. Right, representative image of metaphase spreads from GFP- or YAP-transduced irradiated CGNPs; arrows show examples of broken chromosomes. Table summarizes the number of breaks/cell after analysis of 50 irradiated GFP- or YAP-transduced CGNPs. YAP-transduction was also associated with increased genomic instability in a mouse medulloblastoma cell line (Supplementary Figure 3B). Statistically significant differences are indicated as (**) P < 0.01; (***) P < 0.001.
Figure 4. The ATM-Chk2 DNA damage response pathway is rapidly deactivated in YAP over-expressing cells
(A) Representative western blot analysis of GFP- or YAP-transduced CGNPs at the indicated time points after irradiation. CGNPs over-expressing YAP at one and three hours post-irradiation showed reduced levels of inhibited (Tyr-15 phosphorylated) Cdk1, and higher levels of cyclin B1 indicating increased entry into mitosis. (B) Western blot analysis of GFP- or YAP-transduced CGNPs (left) or NeuroD2-SmoA1 cells (MBCs) (right) at the indicated times after irradiation. YAP-transduced CGNPs show lower levels of phosphorylated ATM and Chk2 3 hours after radiation, compared to GFP-transduced cells. Similar results were observed in irradiated MBCs transduced with either GFP or YAP. Quantification of phosphorylated ATM and Chk2 levels are shown at the bottom. YAP expression did not differentially affect chk1 or p53 levels or phosphorylation.
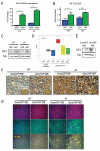
Figure 5. YAP induces expression of IGF2 and activation of Akt
(A) IGF2 was found to be up-regulated in the presence of YAP using microarray analysis, confirmed by quantitative RT-PCR. Irradiation induces IGF2 in GFP- and YAP-infected cells, but levels are significantly higher in YAP-infected CGNPs 3 hours post-irradiation. (B) ELISA analysis of IGF2 production in GFP- or YAP-transduced control or irradiated CGNPs (3h). Statistically significant differences are indicated as (*) P < 0.05; (***) P < 0.001. (C) Western blot analysis of IGF2 protein in GFP- or YAP-transduced control and irradiated CGNPs (3h). (D) Box plot showing IGF2 mRNA expression obtained from exon array profiling of 103 primary human medulloblastomas, 9 fetal and 5 adult human control cerebella. IGF2 is highly expressed specifically in SHH-driven medulloblastomas (T test, p=3.553E-14). IGF1 is not specifically elevated in human SHH-associated medulloblastomas (Supplementary Figure 4). (E) Western blot analysis of IGF2 protein in GFP-SmoA1 and YAP-SmoA1 mouse medulloblastomas. (F) Immunohistochemical staining for IGF2 in medulloblastomas from non-irradiated (left) and irradiated (3h; right) GFP-SmoA1 and YAP-SmoA1 tumor bearing mice. (G) Immunofluorescence analysis of Akt S473 phosphorylation and YAP protein in medulloblastomas from non-irradiated (left) and irradiated (3h; right) GFP-SmoA1 and YAP-SmoA1 tumor bearing mice.
Figure 6. Akt inhibition or IGF2 knock-down abrogate the effects of YAP on DNA repair, proliferation, and survival
(A) Western blot analysis of CGNPs infected with either GFP or YAP, treated with the PI3K inhibitor LY 294002 or with vehicle, irradiated and analyzed three hours after radiation. LY 294002 treatment in YAP-expressing cells restored the levels of phosphorylated-Akt, -ATM, and -Chk2 to the levels observed in GFP-expressing CGNPs. (B) Western blot showing effective knock-down of IGF2 in CGNPs infected with either GFP or YAP, then transduced with lentiviruses targeting IGF2 for short hairpin RNA-mediated knock-down or encoding for a scrambled shRNA sequence. IGF2 knockdown reduces Akt phosphorylation and rescues ATM and Chk2 phosphorylation in YAP-transduced cells 3 hours after irradiation. (C) Immunofluorescence analysis of 53BP1 localization to foci in irradiated GFP- or YAP-transduced CGNPs subsequently infected with retroviruses targeting IGF2 for short hairpin RNA-mediated knock-down or encoding for a scrambled shRNA sequence. Quantification is shown at right. Fewer YAP-transduced CGNPs contained foci 9 and 24 hours post-irradiation compared to GFP-transduced CGNPs. IGF2 knock-down restored the percentage of cells containing DNA damage-induced foci to the levels observed in GFP-expressing CGNPs. (D) Immunofluorescence analysis of cleaved caspase 3 and Ki67 in GFP- or YAP-transduced CGNPs infected with either scrambled or IGF2-targeting retroviruses, three hours after irradiation. IGF2 knock-down restored the levels of proliferation and apoptosis to those observed in GFP-expressing CGNPs. Statistically significant differences are indicated as (*) P < 0.05; (**) P < 0.01; (***) P < 0.001.
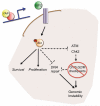
Figure 7. YAP-mediated IGF2 expression drives cell survival, proliferation and genomic instability
Model suggesting the mechanism through which oncogenic YAP expression permits cell proliferation and survival after radiation-induced DNA damage. YAP induces IGF2 expression in CGNPs and medulloblastoma cells. IGF2 activates the PI3K/Akt pathway, promoting survival and increasing proliferation of these cells. In addition, increased activation of Akt leads to faster ATM and Chk2 dephosphorylation after radiation, subsequently deactivating the DNA damage response and removing the G1/S and G2/M checkpoints. Favoring survival, proliferation and genomic instability confers an advantage to YAP-expressing tumor cells. Inhibiting IGF2/Akt signaling in tumors with high YAP expression may prevent recurrence and enable use of lower radiation doses by increasing tumor cell radiosensitivity.
Similar articles
- YB-1 is elevated in medulloblastoma and drives proliferation in Sonic hedgehog-dependent cerebellar granule neuron progenitor cells and medulloblastoma cells.
Dey A, Robitaille M, Remke M, Maier C, Malhotra A, Gregorieff A, Wrana JL, Taylor MD, Angers S, Kenney AM. Dey A, et al. Oncogene. 2016 Aug 11;35(32):4256-68. doi: 10.1038/onc.2015.491. Epub 2016 Jan 4. Oncogene. 2016. PMID: 26725322 Free PMC article. - Sonic hedgehog and insulin-like growth factor signaling synergize to induce medulloblastoma formation from nestin-expressing neural progenitors in mice.
Rao G, Pedone CA, Del Valle L, Reiss K, Holland EC, Fults DW. Rao G, et al. Oncogene. 2004 Aug 12;23(36):6156-62. doi: 10.1038/sj.onc.1207818. Oncogene. 2004. PMID: 15195141 - The miR-17/92 polycistron is up-regulated in sonic hedgehog-driven medulloblastomas and induced by N-myc in sonic hedgehog-treated cerebellar neural precursors.
Northcott PA, Fernandez-L A, Hagan JP, Ellison DW, Grajkowska W, Gillespie Y, Grundy R, Van Meter T, Rutka JT, Croce CM, Kenney AM, Taylor MD. Northcott PA, et al. Cancer Res. 2009 Apr 15;69(8):3249-55. doi: 10.1158/0008-5472.CAN-08-4710. Epub 2009 Apr 7. Cancer Res. 2009. PMID: 19351822 Free PMC article. - Modeling medulloblastoma with genetically engineered mice.
Fults DW. Fults DW. Neurosurg Focus. 2005 Nov 15;19(5):E7. doi: 10.3171/foc.2005.19.5.8. Neurosurg Focus. 2005. PMID: 16398471 Review. - Recent advances in SHH medulloblastoma progression: tumor suppressor mechanisms and the tumor microenvironment.
Tamayo-Orrego L, Charron F. Tamayo-Orrego L, et al. F1000Res. 2019 Oct 29;8:F1000 Faculty Rev-1823. doi: 10.12688/f1000research.20013.1. eCollection 2019. F1000Res. 2019. PMID: 31700613 Free PMC article. Review.
Cited by
- Radiation therapy affects YAP expression and intracellular localization by modulating lamin A/C levels in breast cancer.
La Verde G, Artiola V, Pugliese M, La Commara M, Arrichiello C, Muto P, Netti PA, Fusco S, Panzetta V. La Verde G, et al. Front Bioeng Biotechnol. 2022 Aug 24;10:969004. doi: 10.3389/fbioe.2022.969004. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36091449 Free PMC article. - Liposome-Imipramine Blue Inhibits Sonic Hedgehog Medulloblastoma In Vivo.
MacDonald TJ, Liu J, Yu B, Malhotra A, Munson J, Park JC, Wang K, Fei B, Bellamkonda R, Arbiser J. MacDonald TJ, et al. Cancers (Basel). 2021 Mar 11;13(6):1220. doi: 10.3390/cancers13061220. Cancers (Basel). 2021. PMID: 33799550 Free PMC article. - Medulloblastoma development: tumor biology informs treatment decisions.
Gopalakrishnan V, Tao RH, Dobson T, Brugmann W, Khatua S. Gopalakrishnan V, et al. CNS Oncol. 2015;4(2):79-89. doi: 10.2217/cns.14.58. CNS Oncol. 2015. PMID: 25768332 Free PMC article. Review. - YAP promotes the proliferation of neuroblastoma cells through decreasing the nuclear location of p27Kip1 mediated by Akt.
Shen X, Xu X, Xie C, Liu H, Yang D, Zhang J, Wu Q, Feng W, Wang L, Du L, Xuan L, Meng C, Zhang H, Wang W, Wang Y, Xie T, Huang Z. Shen X, et al. Cell Prolif. 2020 Feb;53(2):e12734. doi: 10.1111/cpr.12734. Epub 2019 Dec 20. Cell Prolif. 2020. PMID: 31863533 Free PMC article. - PTEN lipid phosphatase inactivation links the hippo and PI3K/Akt pathways to induce gastric tumorigenesis.
Xu W, Yang Z, Xie C, Zhu Y, Shu X, Zhang Z, Li N, Chai N, Zhang S, Wu K, Nie Y, Lu N. Xu W, et al. J Exp Clin Cancer Res. 2018 Aug 22;37(1):198. doi: 10.1186/s13046-018-0795-2. J Exp Clin Cancer Res. 2018. PMID: 30134988 Free PMC article.
References
- Berman DM, Karhadkar SS, Hallahan AR, Pritchard JI, Eberhart CG, Watkins DN, et al. Medulloblastoma growth inhibition by hedgehog pathway blockade. Science. 2002;297:1559–1561. - PubMed
- Bertini E, Oka T, Sudol M, Strano S, Blandino G. YAP: at the crossroad between transformation and tumor suppression. Cell Cycle. 2009;8:49–57. - PubMed
- Brunet A, Datta SR, Greenberg ME. Transcription-dependent and - independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol. 2001;11:297–305. - PubMed
- Burgering BM, Kops GJ. Cell cycle and death control: long live Forkheads. Trends Biochem Sci. 2002;27:352–360. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous