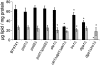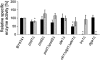Metabolic link between phosphatidylethanolamine and triacylglycerol metabolism in the yeast Saccharomyces cerevisiae - PubMed (original) (raw)
Metabolic link between phosphatidylethanolamine and triacylglycerol metabolism in the yeast Saccharomyces cerevisiae
Susanne E Horvath et al. Biochim Biophys Acta. 2011 Dec.
Abstract
In the yeast Saccharomyces cerevisiae triacylglycerols (TAG) are synthesized by the acyl-CoA dependent acyltransferases Dga1p, Are1p, Are2p and the acyl-CoA independent phospholipid:diacylglycerol acyltransferase (PDAT) Lro1p which uses phosphatidylethanolamine (PE) as a preferred acyl donor. In the present study we investigated a possible link between TAG and PE metabolism by analyzing the contribution of the four different PE biosynthetic pathways to TAG formation, namely de novo PE synthesis via Psd1p and Psd2p, the CDP-ethanolamine (CDP-Etn) pathway and lyso-PE acylation by Ale1p. In cells grown on the non-fermentable carbon source lactate supplemented with 5mM ethanolamine (Etn) the CDP-Etn pathway contributed most to the cellular TAG level, whereas mutations in the other pathways displayed only minor effects. In cki1∆dpl1∆eki1∆ mutants bearing defects in the CDP-Etn pathway both the cellular and the microsomal levels of PE were markedly decreased, whereas in other mutants of PE biosynthetic routes depletion of this aminoglycerophospholipid was less pronounced in microsomes. This observation is important because Lro1p similar to the enzymes of the CDP-Etn pathway is a component of the ER. We conclude from these results that in cki1∆dpl1∆eki1∆ insufficient supply of PE to the PDAT Lro1p was a major reason for the strongly reduced TAG level. Moreover, we found that Lro1p activity was markedly decreased in cki1∆dpl1∆eki1∆, although transcription of LRO1 was not affected. Our findings imply that (i) TAG and PE syntheses in the yeast are tightly linked; and (ii) TAG formation by the PDAT Lro1p strongly depends on PE synthesis through the CDP-Etn pathway. Moreover, it is very likely that local availability of PE in microsomes is crucial for TAG synthesis through the Lro1p reaction.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures
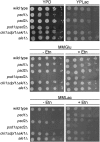
Fig. 1
Growth of yeast strains with defects in phosphatidylethanolamine biosynthesis depends on the carbon sources. Cell suspensions of strains listed in the figure were spotted at dilutions (1, 1/10, 1/100, 1/1000, 1/10,000) on YPD, YPLac, MMGlu and MMLac with or without 5 mM ethanolamine. Incubation was carried out at 30 °C. YPD, complex glucose media; YPLac, complex lactate media; MMGlu, minimal glucose media; MMLac, minimal lactate media; Etn, ethanolamine.
Fig. 2
Neutral lipid composition of wild type and mutants defective in either phosphatidylethanolamine or triacylglycerol biosynthesis grown to stationary phase. Amounts of triacylglycerols (black bars) and ergosteryl esters (gray bars) in μg lipid per mg protein were measured in strains as indicated. Data are mean values of 3 independent experiments with error bars indicating the standard deviation. Significance was calculated by Student's _t_-test (two tailed, unpaired). Values indicated by * correspond to P < 0.05 and were defined to be significant.
Fig. 3
Relative activities of triacylglycerol synthesizing enzymes in vitro. Acyl-CoA:diacylglycerol acyltransferase activity (Dga1p) (gray bars) was measured in vitro using total cell-free homogenate. Phospholipid:diacylglycerol acyltransferase activity (Lro1p) (black bars) was measured using 100,000 × g microsomes. The specific activity of Lro1p and Dga1p in wild type was set to 100%, and data for mutant strains were calculated accordingly. As negative control, phospholipid:diacylglycerol acyltransferase was measured in lro1Δ, and acyl-CoA:diacylglycerol acyltransferase in dga1Δ. Data are mean values of 3 independent experiments with error bars indicating the standard deviation. Significance was calculated by Student's _t_-test (two tailed, unpaired). Values indicated by * correspond to P < 0.05 and were defined to be significant.
Fig. 4
Gene expression levels of LRO1, DGA1 and ACT1 from wild type and strains defective in phosphatidylethanolamine and triacylglycerol biosynthesis. Strains listed in the figure were tested by RT-PCR. ACT1 (actin) was used as a loading control and lro1Δ and dga1Δ were used as negative control for LRO1 and DGA1 expression.
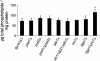
Fig. 5
Amounts of total phospholipids from wild type and strains defective in phosphatidylethanolamine and triacylglycerol biosynthesis grown to the stationary phase. The amounts of total phospholipids in μg lipid per mg protein were measured in strains as indicated. Data are mean values of 3 independent experiments with error bars indicating the standard deviation. Significance was calculated by Student's _t_-test (two tailed, unpaired). Values indicated by * correspond to P < 0.05 and were defined to be significant.
Similar articles
- Synthesis of triacylglycerols by the acyl-coenzyme A:diacyl-glycerol acyltransferase Dga1p in lipid particles of the yeast Saccharomyces cerevisiae.
Sorger D, Daum G. Sorger D, et al. J Bacteriol. 2002 Jan;184(2):519-24. doi: 10.1128/JB.184.2.519-524.2002. J Bacteriol. 2002. PMID: 11751830 Free PMC article. - Phosphatidylethanolamine synthesized by four different pathways is supplied to the plasma membrane of the yeast Saccharomyces cerevisiae.
Schuiki I, Schnabl M, Czabany T, Hrastnik C, Daum G. Schuiki I, et al. Biochim Biophys Acta. 2010 Apr;1801(4):480-6. doi: 10.1016/j.bbalip.2009.12.008. Epub 2009 Dec 28. Biochim Biophys Acta. 2010. PMID: 20044027 - Contribution of different pathways to the supply of phosphatidylethanolamine and phosphatidylcholine to mitochondrial membranes of the yeast Saccharomyces cerevisiae.
Bürgermeister M, Birner-Grünberger R, Nebauer R, Daum G. Bürgermeister M, et al. Biochim Biophys Acta. 2004 Nov 8;1686(1-2):161-8. doi: 10.1016/j.bbalip.2004.09.007. Biochim Biophys Acta. 2004. PMID: 15522832 - Triacylglycerol biosynthesis in yeast.
Sorger D, Daum G. Sorger D, et al. Appl Microbiol Biotechnol. 2003 May;61(4):289-99. doi: 10.1007/s00253-002-1212-4. Epub 2003 Jan 29. Appl Microbiol Biotechnol. 2003. PMID: 12743757 Review. - Formation and mobilization of neutral lipids in the yeast Saccharomyces cerevisiae.
Wagner A, Daum G. Wagner A, et al. Biochem Soc Trans. 2005 Nov;33(Pt 5):1174-7. doi: 10.1042/BST20051174. Biochem Soc Trans. 2005. PMID: 16246075 Review.
Cited by
- Regulation of the yeast triacylglycerol lipase TGl3p by formation of nonpolar lipids.
Schmidt C, Athenstaedt K, Koch B, Ploier B, Daum G. Schmidt C, et al. J Biol Chem. 2013 Jul 5;288(27):19939-48. doi: 10.1074/jbc.M113.459610. Epub 2013 May 14. J Biol Chem. 2013. PMID: 23673660 Free PMC article. - The Lipid Profile of the Endomyces magnusii Yeast upon the Assimilation of the Substrates of Different Types and upon Calorie Restriction.
Deryabina YI, Kokoreva AS, Klein OI, Gessler NN, Isakova EP. Deryabina YI, et al. J Fungi (Basel). 2022 Nov 21;8(11):1233. doi: 10.3390/jof8111233. J Fungi (Basel). 2022. PMID: 36422054 Free PMC article. - Engineering yeast phospholipid metabolism for de novo oleoylethanolamide production.
Liu Y, Liu Q, Krivoruchko A, Khoomrung S, Nielsen J. Liu Y, et al. Nat Chem Biol. 2020 Feb;16(2):197-205. doi: 10.1038/s41589-019-0431-2. Epub 2019 Dec 16. Nat Chem Biol. 2020. PMID: 31844304 - Lipid droplet-mediated lipid and protein homeostasis in budding yeast.
Graef M. Graef M. FEBS Lett. 2018 Apr;592(8):1291-1303. doi: 10.1002/1873-3468.12996. Epub 2018 Feb 16. FEBS Lett. 2018. PMID: 29397034 Free PMC article. Review. - Phosphatidylserine and GTPase activation control Cdc42 nanoclustering to counter dissipative diffusion.
Sartorel E, Ünlü C, Jose M, Massoni-Laporte A, Meca J, Sibarita JB, McCusker D. Sartorel E, et al. Mol Biol Cell. 2018 Jun 1;29(11):1299-1310. doi: 10.1091/mbc.E18-01-0051. Epub 2018 Apr 18. Mol Biol Cell. 2018. PMID: 29668348 Free PMC article.
References
- Zweytick D., Athenstaedt K., Daum G. Intracellular lipid particles of eukaryotic cells. Biochim. Biophys. Acta. 2000;1469:101–120. - PubMed
- Czabany T., Wagner A., Zweytick D., Lohner K., Leitner E., Ingolic E., Daum G. Structural and biochemical properties of lipid particles from the yeast Saccharomyces cerevisiae. J. Biol. Chem. 2008;283:17065–17074. - PubMed
- Zweytick D., Leitner E., Kohlwein S.D., Yu C., Rothblatt J., Daum G. Contribution of Are1p and Are2p to steryl ester synthesis in the yeast Saccharomyces cerevisiae. Eur. J. Biochem. 2000;267:1075–1082. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases