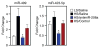Therapeutic inhibition of miR-208a improves cardiac function and survival during heart failure - PubMed (original) (raw)
Therapeutic inhibition of miR-208a improves cardiac function and survival during heart failure
Rusty L Montgomery et al. Circulation. 2011.
Abstract
Background: Diastolic dysfunction in response to hypertrophy is a major clinical syndrome with few therapeutic options. MicroRNAs act as negative regulators of gene expression by inhibiting translation or promoting degradation of target mRNAs. Previously, we reported that genetic deletion of the cardiac-specific miR-208a prevents pathological cardiac remodeling and upregulation of Myh7 in response to pressure overload. Whether this miRNA might contribute to diastolic dysfunction or other forms of heart disease is currently unknown.
Methods and results: Here, we show that systemic delivery of an antisense oligonucleotide induces potent and sustained silencing of miR-208a in the heart. Therapeutic inhibition of miR-208a by subcutaneous delivery of antimiR-208a during hypertension-induced heart failure in Dahl hypertensive rats dose-dependently prevents pathological myosin switching and cardiac remodeling while improving cardiac function, overall health, and survival. Transcriptional profiling indicates that antimiR-208a evokes prominent effects on cardiac gene expression; plasma analysis indicates significant changes in circulating levels of miRNAs on antimiR-208a treatment.
Conclusions: These studies indicate the potential of oligonucleotide-based therapies for modulating cardiac miRNAs and validate miR-208 as a potent therapeutic target for the modulation of cardiac function and remodeling during heart disease progression.
Figures
Figure 1
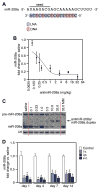
Systemic delivery of antimiR-208a induces potent and sustained silencing of miR-208 in the heart. A, Locked nucleic acid (LNA)–modified antisense oligonucleotide chemistry directed against the 5′end of miR-208a (antimiR-208a). B, Real-time polymerase chain reaction analysis on murine hearts 1 week after intravenous delivery of increasing doses of antimiR-208a shows a dose-dependent reduction in miR-208a levels. C, Northern blot analysis on total RNA from murine hearts 1 week after intravenous delivery of antimiR-208a shows a dose-dependent reduction in miR-208a detection, whereas a control mismatch chemistry (MM) has no effect on miR-208a. U6 serves as a loading control. D, Real-time polymerase chain reaction analysis on cardiac tissue collected at the indicated time points shows that intravenous, intraperitoneal, or subcutaneous delivery of 25 mg/kg antimiR-208a induces potent silencing of miR-208a. In B and D, error bars depict SEM (n=4 for each time point and dose).
Figure 2
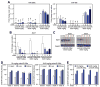
miR-208a silencing reduces miR-499 and Myh7. A, Real-time polymerase chain reaction analysis shows that antimiR-208a potently reduces cardiac levels of miR-208a up to 6 weeks after injection, which leads to a time-dependent reduction in miR-499. Dosing with an anti-miR against both miR-208a and miR-499 induces a more rapid reduction in cardiac levels of both miR-208a and miR-499. Control anti-miR shows no robust effect on miR-208a or miR-499 expression. B, Myh7 is reduced 4 weeks after miR-208a inhibition, whereas inhibition of miR-208a and miR-499 reduces Myh7 after 2 weeks, as shown by real-time polymerase chain reaction. Control anti-miR shows no robust effect on Myh7 expression. C, Western blot analysis for Myh7 showing reduced Myh7 expression at the indicated time points after antimiR-208a or antimiR-208a/-499 treatment. GAPDH serves as a loading control. Each lane is a representative animal from B. D and E, Tissue distribution analysis indicates that antimiR-208a is detectable in heart, liver, kidney (D), and plasma (E) up to 6 weeks after injection. In A, B, D, and E, error bars depict the SEM (n=4 for each time point and dose). In A and B, *P<0.05 vs saline at the same time point; #P<0.05 vs control anti-miR at the same time point. In D, *P<0.05 vs week 1 for the same tissue.
Figure 3
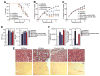
Therapeutic silencing of miR-208a is beneficial during heart failure. A, Kaplan-Meier survival curves in the Dahl hypertensive rat model show a pronounced decrease in survival in response to an 8% high-salt (HS) diet for both the HS/saline and HS/control groups, which is significantly improved in response to antimiR-208a treatment. Rats were dosed every 2 weeks at 25 mg/kg starting 1 week after the HS diet. *_P_=0.0038 vs HS/saline; #P<0.0001 vs HS/control. B, Body weight analysis indicates that Dahl hypertensive rats on an 8% HS diet exhibit reduced weight gain compared with animals on a low-salt (LS) diet, whereas HS/antimiR-208a–treated rats show a significantly better maintenance in weight gain. For A and B, n=6 for LS/saline; n=15 for HS/saline and HS/control; and n=14 for HS/antimiR-208a. n Indicates total survivors remaining at week 8 after the diet. C, Body weight analysis of Dahl rats on the 4% HS diet shows significant reductions in weight gain compared with LS diet controls, whereas both 5- and 25-mg/kg injections every 2 weeks are sufficient to maintain weight gain comparable to that in animals on a normal diet. D, Echocardiography measurements indicate that the increase in isovolumic relaxation time (IVRT) and decrease in mitral valve early to active filling velocity ratio (MV E/A) in response to a 4% HS diet are significantly improved in response to antimiR-208a treatment 8 weeks after the start of the diet. For C and D, n=10 for all groups. E, Representative images of hematoxylin and eosin (H&E)– and Picrosirius Red–stained left ventricular histological sections indicate an increase in cardiomyocyte hypertrophy and perivascular fibrosis in response to the 4% HS diet for 8 weeks, whereas both parameters are reduced in response to antimiR-208a treatment. H&E, scale bar = 50 _μ_m; Picrosirius Red, scale bar =100 _μ_m. F, Bar graph representation of histological quantification showing significantly less hypertrophy and fibrosis in the presence of antimiR-208a. In D and F, error bars depict the SEM. *P<0.05 vs HS/saline; #P<0.05 vs LS/saline.
Figure 4
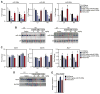
AntimiR-208a treatment reduces miR-499 and Myh7 in Dahl salt-sensitive rats. All analyses were performed 8 weeks after the start of a 4% high-salt (HS) diet and 7 weeks after the start of anti-miR treatment (n=10 for all groups in A and C). In B and D, each lane is a representative animal from the n=10 group. Rats were dosed every 2 weeks at 25 mg/kg (control) or the indicated dose of antimiR-208a starting 1 week after the HS diet. Red lines on graphs separate the groups. A, Real-time polymerase chain reaction analysis indicates a dose-dependent reduction of miR-208a in both the left ventricle (LV) and right ventricle (RV), which corresponds to a dose-dependent decrease in miR-499. Although miR-208b is increased in response to the HS diet, antimiR-208a significantly blunts this response. Administration of a scrambled control chemistry had no effect on the expression of miR-208a, miR-499, or miR-208b. B, Regulation of miR-499 and miR-208b in response to antimiR-208a treatment shown by Northern blot analysis. C, Real-time polymerase chain reaction analysis shows that the HS diet reduces Myh6 and increases Myh7. AntimiR-208a treatment dose-dependently increases Myh6 expression and reduces Myh7b expression. The HS diet–induced increase in Myh7 is dose-dependently reduced by anti–miR-208a. D, Western blot analysis for Myh7 from ventricular tissue confirms the dose-dependent reduction in response to antimiR-208a treatment. GAPDH is used as a loading control. E, Quantification of HP1_β_ Western blot showing miR-208a target derepression in the presence of antimiR-208a. The full blot in shown in Figure V in the online-only Data Supplement. In A and C, error bars depict the SEM. *P<0.05 vs HS/saline, #P<0.05 vs LS/saline.
Figure 5
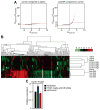
AntimiR-208a regulates a specific subset of genes in Dahl salt-sensitive rats. A, Microarray analysis was performed from left ventricular RNA from high salt (HS)/saline, HS/25 mg/kg antimiR-208a, and HS/control at 8 weeks after the start of a 4% HS diet and 7 weeks after the start of anti-miR treatment. Differential expression graphs show the numbers of genes that are differentially expressed when comparing gene expression in hearts of control-treated animals and saline-treated animals (left) or antimiR-208a–treated animals with control-treated animals (right). Dpredicted is the bioinformatically computed reference distribution for the 13 518 transcripts detected on the array. The transcripts highlighted in red are significantly different and due to nonrandom difference, as calculated by Dobserved-Dpredicted. B, Hierarchical clustering and heat map visualization of the 131 differentially expressed genes between control oligo– and antimiR-208a–treated hearts show clustering of treatment groups and few gene changes in the saline-treated vs the control oligo–treated animals. A full list of transcripts in given in Table IV in the online-only Data Supplement. C, Quantification of Dynlt1 Western blot showing miR-208a target derepression in the presence of antimiR-208a. A full blot in given in Figure VIII in the online-only Data Supplement.
Figure 6
miR-499 in plasma serves a biomarker for antimiR-208a efficacy. Real-time polymerase chain reaction (PCR) analysis of the plasma samples indicates an increase in miR-499 in response to a high-salt (HS) diet, whereas antimiR-208a significantly lowers the detection of miR-499 in plasma 8 weeks after the start of a 4% HS diet and 7 weeks after the start of anti-miR treatment. Further miRNA analysis indicates a decrease in plasma-detectable miR-423–5p in response to antimiR-208a. *P<0.05 vs HS saline (n=10 per group).
Similar articles
- Early dysregulation of cardiac-specific microRNA-208a is linked to maladaptive cardiac remodelling in diabetic myocardium.
Rawal S, Nagesh PT, Coffey S, Van Hout I, Galvin IF, Bunton RW, Davis P, Williams MJA, Katare R. Rawal S, et al. Cardiovasc Diabetol. 2019 Jan 29;18(1):13. doi: 10.1186/s12933-019-0814-4. Cardiovasc Diabetol. 2019. PMID: 30696455 Free PMC article. - Plasma microRNAs serve as biomarkers of therapeutic efficacy and disease progression in hypertension-induced heart failure.
Dickinson BA, Semus HM, Montgomery RL, Stack C, Latimer PA, Lewton SM, Lynch JM, Hullinger TG, Seto AG, van Rooij E. Dickinson BA, et al. Eur J Heart Fail. 2013 Jun;15(6):650-9. doi: 10.1093/eurjhf/hft018. Epub 2013 Feb 6. Eur J Heart Fail. 2013. PMID: 23388090 - MicroRNA-208a is a regulator of cardiac hypertrophy and conduction in mice.
Callis TE, Pandya K, Seok HY, Tang RH, Tatsuguchi M, Huang ZP, Chen JF, Deng Z, Gunn B, Shumate J, Willis MS, Selzman CH, Wang DZ. Callis TE, et al. J Clin Invest. 2009 Sep;119(9):2772-86. doi: 10.1172/JCI36154. Epub 2009 Aug 10. J Clin Invest. 2009. PMID: 19726871 Free PMC article. - The emerging role of miR-208a in the heart.
Oliveira-Carvalho V, Carvalho VO, Bocchi EA. Oliveira-Carvalho V, et al. DNA Cell Biol. 2013 Jan;32(1):8-12. doi: 10.1089/dna.2012.1787. Epub 2012 Nov 2. DNA Cell Biol. 2013. PMID: 23121236 Review. - miR-208a in Cardiac Hypertrophy and Remodeling.
Huang XH, Li JL, Li XY, Wang SX, Jiao ZH, Li SQ, Liu J, Ding J. Huang XH, et al. Front Cardiovasc Med. 2021 Dec 9;8:773314. doi: 10.3389/fcvm.2021.773314. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34957257 Free PMC article. Review.
Cited by
- Epigenetics in heart failure phenotypes.
Berezin A. Berezin A. BBA Clin. 2016 May 30;6:31-7. doi: 10.1016/j.bbacli.2016.05.005. eCollection 2016 Dec. BBA Clin. 2016. PMID: 27335803 Free PMC article. Review. - Understanding cardiomyopathy phenotypes based on the functional impact of mutations in the myosin motor.
Moore JR, Leinwand L, Warshaw DM. Moore JR, et al. Circ Res. 2012 Jul 20;111(3):375-85. doi: 10.1161/CIRCRESAHA.110.223842. Circ Res. 2012. PMID: 22821910 Free PMC article. Review. - How cardiomyocytes sense pathophysiological stresses for cardiac remodeling.
Haque ZK, Wang DZ. Haque ZK, et al. Cell Mol Life Sci. 2017 Mar;74(6):983-1000. doi: 10.1007/s00018-016-2373-0. Epub 2016 Oct 6. Cell Mol Life Sci. 2017. PMID: 27714411 Free PMC article. Review. - Cardiac Metabolism and MiRNA Interference.
Sumaiya K, Ponnusamy T, Natarajaseenivasan K, Shanmughapriya S. Sumaiya K, et al. Int J Mol Sci. 2022 Dec 20;24(1):50. doi: 10.3390/ijms24010050. Int J Mol Sci. 2022. PMID: 36613495 Free PMC article. Review. - A journey from microenvironment to macroenvironment: the role of metaflammation and epigenetic changes in cardiorenal disease.
Kanbay M, Yerlikaya A, Sag AA, Ortiz A, Kuwabara M, Covic A, Wiecek A, Stenvinkel P, Afsar B. Kanbay M, et al. Clin Kidney J. 2019 Sep 18;12(6):861-870. doi: 10.1093/ckj/sfz106. eCollection 2019 Dec. Clin Kidney J. 2019. PMID: 31807301 Free PMC article.
References
- Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358:1370–1380. - PubMed
- Frey N, Katus HA, Olson EN, Hill JA. Hypertrophy of the heart: a new therapeutic target? Circulation. 2004;109:1580–1589. - PubMed
- Vanderheyden M, Mullens W, Delrue L, Goethals M, de Bruyne B, Wijns W, Geelen P, Verstreken S, Wellens F, Bartunek J. Myocardial gene expression in heart failure patients treated with cardiac resynchronization therapy responders versus nonresponders. J Am Coll Cardiol. 2008;51:129–136. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL077439/HL/NHLBI NIH HHS/United States
- R01 AR040339/AR/NIAMS NIH HHS/United States
- R01 HL111665/HL/NHLBI NIH HHS/United States
- P50 HL061033/HL/NHLBI NIH HHS/United States
- S10 RR019137/RR/NCRR NIH HHS/United States
- R01 HL093039/HL/NHLBI NIH HHS/United States
- R37 HL053351/HL/NHLBI NIH HHS/United States
- R01 HL083371/HL/NHLBI NIH HHS/United States
- R01 HL061544/HL/NHLBI NIH HHS/United States
- R01 HL053351/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical