Exploiting bacterial DNA gyrase as a drug target: current state and perspectives - PubMed (original) (raw)
Review
Exploiting bacterial DNA gyrase as a drug target: current state and perspectives
Frédéric Collin et al. Appl Microbiol Biotechnol. 2011 Nov.
Abstract
DNA gyrase is a type II topoisomerase that can introduce negative supercoils into DNA at the expense of ATP hydrolysis. It is essential in all bacteria but absent from higher eukaryotes, making it an attractive target for antibacterials. The fluoroquinolones are examples of very successful gyrase-targeted drugs, but the rise in bacterial resistance to these agents means that we not only need to seek new compounds, but also new modes of inhibition of this enzyme. We review known gyrase-specific drugs and toxins and assess the prospects for developing new antibacterials targeted to this enzyme.
Figures
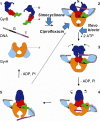
Fig. 1
Gyrase mechanism (adapted from Costenaro et al. 2007). 1 Free states of the proteins and DNA. 2 Wrapping of the DNA around the enzyme presents the T segment over the G segment. 3 Upon ATP binding, GyrB dimerises, captures the T segment, and the G segment is transiently cleaved. 4 Hydrolysis of one ATP allows GyrB to rotate, the GyrA opening to widen and the transport of the T segment through the cleaved G segment. 5 Religation of the G segment results in the introduction of two negative supercoils into DNA; release of the T segment and hydrolysis of the second ATP resets the enzyme. Stars indicate the positions of the active-site residues for DNA cleavage, and the circle indicates the ATP-binding pocket. Colour code of the domains: GyrA59 (N-terminal domain; NTD), orange; GyrA-CTD, cyan; GyrB43 (NTD), blue; Toprim, red; Tail, green; G segment, black; T segment, purple. The approximate points of action of simocyclinone, novobiocin and ciprofloxacin are shown. N, D and E indicate the locations of the N gate, DNA gate and exit gate, respectively (reprinted with permission from Elsevier; Costenaro et al. 2007)
Fig. 2
Quinolones. a Examples of fluoroquinolones and their precursor nalidixic acid. b Structure of the topo IV–fluoroquinolone complex (Laponogov et al. 2009). Upper: front view of the topo IV ParC55 and ParE30 proteins complexed with the G segment DNA and moxifloxacin, shown in cartoon representation. DNA is green, the TOPRIM domain (ParE30) is in yellow and the ParC55 is blue. The moxifloxacin molecules are shown in red. Lower: close up of the quinolone–topo IV cleavage complex in cartoon representation. The active site tyrosines (Tyr118) are in orange and the residues responsible for drug resistance upon mutation are shown in yellow (b is reproduced with permission from Nature Publishing Group; Laponogov et al. 2009)
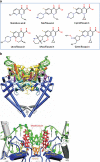
Fig. 3
Aminocoumarins. a Classical aminocoumarins. b Upper: structure of the N-terminal domain of GyrB (GyrB43) complexed with an ATP analogue (Wigley et al. 1991); the protein is in cartoon representation with the two monomers in shades of green and shades of yellow; the nucleotide is in space-filling representation (reproduced with permission (Maxwell and Lawson 2003)). Lower: structure of the N-terminal sub-domain of GyrB (GyrB24) complexed with novobiocin (blue) showing the overlap with bound ATP (Lewis et al. 1996a). c Simocyclinone D8. d Upper: structure of the complex between simocyclinone D8 and the N-terminal domain of GyrA (GyrA59) (Edwards et al. 2009). The protein dimer is shown in blue in cartoon representation and the drug in yellow in stick representation (reprinted with permission from AAAS; Edwards et al. 2009) Lower: detail of the simocyclinone-binding pockets in GyrA showing two alternative modes of interaction (Edwards et al. ; Edwards et al. 2011). The blue subunit has the two simocyclinone pockets occupied by two different simocyclinone molecules; the brown subunit has a single simocyclinone molecule bridging both pockets (reprinted with permission from the American Chemical Society; Edwards et al. 2011)
Fig. 3
Aminocoumarins. a Classical aminocoumarins. b Upper: structure of the N-terminal domain of GyrB (GyrB43) complexed with an ATP analogue (Wigley et al. 1991); the protein is in cartoon representation with the two monomers in shades of green and shades of yellow; the nucleotide is in space-filling representation (reproduced with permission (Maxwell and Lawson 2003)). Lower: structure of the N-terminal sub-domain of GyrB (GyrB24) complexed with novobiocin (blue) showing the overlap with bound ATP (Lewis et al. 1996a). c Simocyclinone D8. d Upper: structure of the complex between simocyclinone D8 and the N-terminal domain of GyrA (GyrA59) (Edwards et al. 2009). The protein dimer is shown in blue in cartoon representation and the drug in yellow in stick representation (reprinted with permission from AAAS; Edwards et al. 2009) Lower: detail of the simocyclinone-binding pockets in GyrA showing two alternative modes of interaction (Edwards et al. ; Edwards et al. 2011). The blue subunit has the two simocyclinone pockets occupied by two different simocyclinone molecules; the brown subunit has a single simocyclinone molecule bridging both pockets (reprinted with permission from the American Chemical Society; Edwards et al. 2011)
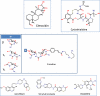
Fig. 4
Other small molecule gyrase inhibitors
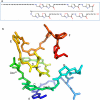
Fig. 5
Microcin B17. a Primary structure. b Proposed 3-D structure (adapted from (Parks et al. 2007) with permission from Elsevier). Letters A–F indicate the heterocycles, and key amino acids are numbered
Fig. 6
CcdB. a Model of gyrase inhibition by CcdB. The proteins and DNA come together (2) to form the holoenzyme with the G segment binding across the DNA gate (3). The DNA is wrapped around GyrA presenting the T segment over the G segment (4). Upon ATP binding, the GyrB monomers dimerise (5), the G segment is cleaved and the DNA gate opens. Either (6a) ‘top-down’ passage of the T segment occurs upon hydrolysis of a single ATP or (6b) CcdB accesses its binding site to stabilise the cleavage complex. A futile ATP hydrolysis/ATP binding cycle can occur (Kampranis et al. 1999b). Several cycles of (5) to (6a) transition may occur prior to CcdB binding. In the normal cycle, the DNA gate closes, the G segment is religated and the T segment passes out through the bottom gate, with hydrolysis of the second ATP resetting the enzyme (7) (reproduced from (Smith and Maxwell 2006) with permission from Oxford University Press.) b Complex of CcdB and GyrA14 and models: (a) Gyr-A14: CcdB complex viewed along its twofold axis. The two CcdB monomers are drawn in orange and red and the two GyrA14 monomers in two shades of blue. (b) Same as (a), but rotated 90°. (c) Model of closed GyrA59 (blue) with CcdB (green) docked onto it by superposition of the GyrA14:CcdB complex on the crystal structure of GyrA59. Residues Arg462 of both monomers of GyrA59 are highlighted. Strong steric overlaps (red) with the CAP domains are observed. (d) Model of the GyrA59:CcdB complex with GyrA59 in its open conformation. The open conformation of GyrA59 was modelled according to the corresponding yeast topoisomerase II structure (PDB entry 1BGW). In this model, there is no steric overlap between CcdB and GyrA59 (reproduced from (Dao-Thi et al. 2005) with permission from Elsevier)
Fig. 7
MfpA complexed with GyrA (Hegde et al. 2005). The MfpA protein (green) is shown docked into the structure of the E. coli GyrA N-terminal domain. Upper: side view; lower: top view (reproduced with permission from AAAS; Hegde et al. 2005)
Similar articles
- Gyrase and Topoisomerase IV: Recycling Old Targets for New Antibacterials to Combat Fluoroquinolone Resistance.
Collins JA, Osheroff N. Collins JA, et al. ACS Infect Dis. 2024 Apr 12;10(4):1097-1115. doi: 10.1021/acsinfecdis.4c00128. Epub 2024 Apr 2. ACS Infect Dis. 2024. PMID: 38564341 Free PMC article. Review. - Fluoroquinolone-gyrase-DNA complexes: two modes of drug binding.
Mustaev A, Malik M, Zhao X, Kurepina N, Luan G, Oppegard LM, Hiasa H, Marks KR, Kerns RJ, Berger JM, Drlica K. Mustaev A, et al. J Biol Chem. 2014 May 2;289(18):12300-12. doi: 10.1074/jbc.M113.529164. Epub 2014 Feb 4. J Biol Chem. 2014. PMID: 24497635 Free PMC article. - Bacterial topoisomerases, anti-topoisomerases, and anti-topoisomerase resistance.
Hooper DC. Hooper DC. Clin Infect Dis. 1998 Aug;27 Suppl 1:S54-63. doi: 10.1086/514923. Clin Infect Dis. 1998. PMID: 9710672 Review. - New structure-activity relationships of the quinolone antibacterials using the target enzyme. The development and application of a DNA gyrase assay.
Domagala JM, Hanna LD, Heifetz CL, Hutt MP, Mich TF, Sanchez JP, Solomon M. Domagala JM, et al. J Med Chem. 1986 Mar;29(3):394-404. doi: 10.1021/jm00153a015. J Med Chem. 1986. PMID: 3005575 - The interaction of drugs with DNA gyrase: a model for the molecular basis of quinolone action.
Heddle JG, Barnard FM, Wentzell LM, Maxwell A. Heddle JG, et al. Nucleosides Nucleotides Nucleic Acids. 2000 Aug;19(8):1249-64. doi: 10.1080/15257770008033048. Nucleosides Nucleotides Nucleic Acids. 2000. PMID: 11097055 Review.
Cited by
- An Insight into Rational Drug Design: The Development of In-House Azole Compounds with Antimicrobial Activity.
Ungureanu D, Oniga O, Moldovan C, Ionuț I, Marc G, Stana A, Pele R, Duma M, Tiperciuc B. Ungureanu D, et al. Antibiotics (Basel). 2024 Aug 13;13(8):763. doi: 10.3390/antibiotics13080763. Antibiotics (Basel). 2024. PMID: 39200063 Free PMC article. Review. - Single-nucleotide-resolution mapping of DNA gyrase cleavage sites across the Escherichia coli genome.
Sutormin D, Rubanova N, Logacheva M, Ghilarov D, Severinov K. Sutormin D, et al. Nucleic Acids Res. 2019 Feb 20;47(3):1373-1388. doi: 10.1093/nar/gky1222. Nucleic Acids Res. 2019. PMID: 30517674 Free PMC article. - Genome-wide assessment of antimicrobial tolerance in Yersinia pseudotuberculosis under ciprofloxacin stress.
Willcocks S, Huse KK, Stabler R, Oyston PCF, Scott A, Atkins HS, Wren BW. Willcocks S, et al. Microb Genom. 2019 Nov;5(11):e000304. doi: 10.1099/mgen.0.000304. Microb Genom. 2019. PMID: 31580793 Free PMC article. - Cryo-EM structure of the complete E. coli DNA gyrase nucleoprotein complex.
Vanden Broeck A, Lotz C, Ortiz J, Lamour V. Vanden Broeck A, et al. Nat Commun. 2019 Oct 30;10(1):4935. doi: 10.1038/s41467-019-12914-y. Nat Commun. 2019. PMID: 31666516 Free PMC article. - Structural insights into the gating of DNA passage by the topoisomerase II DNA-gate.
Chen SF, Huang NL, Lin JH, Wu CC, Wang YR, Yu YJ, Gilson MK, Chan NL. Chen SF, et al. Nat Commun. 2018 Aug 6;9(1):3085. doi: 10.1038/s41467-018-05406-y. Nat Commun. 2018. PMID: 30082834 Free PMC article.
References
- Andersen NR, Rasmussen PR. The constitution of clerocidin a new antibiotic isolated from Oidiodendron truncatum. Tetrahedron Letters. 1984;25(4):465–468. doi: 10.1016/S0040-4039(00)99912-X. - DOI
- Angehrn P, Buchmann S, Funk C, Goetschi E, Gmuender H, Hebeisen P, Kostrewa D, Link H, Luebbers T, Masciadri R, Nielsen J, Reindl P, Ricklin F, Schmitt-Hoffmann A, Theil FP. New antibacterial agents derived from the DNA gyrase inhibitor cyclothialidine. J Med Chem. 2004;47(6):1487–1513. doi: 10.1021/jm0310232. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical