Mesenteric fat as a source of C reactive protein and as a target for bacterial translocation in Crohn's disease - PubMed (original) (raw)
doi: 10.1136/gutjnl-2011-300370. Epub 2011 Sep 21.
Florent Gonzalez, Laurent Dubuquoy, Christel Rousseaux, Caroline Dubuquoy, Cécilia Decourcelle, Alain Saudemont, Mickael Tachon, Elodie Béclin, Marie-Françoise Odou, Christel Neut, Jean-Frédéric Colombel, Pierre Desreumaux
Affiliations
- PMID: 21940721
- PMCID: PMC3230831
- DOI: 10.1136/gutjnl-2011-300370
Mesenteric fat as a source of C reactive protein and as a target for bacterial translocation in Crohn's disease
Laurent Peyrin-Biroulet et al. Gut. 2012 Jan.
Abstract
Objective: Mesenteric fat hyperplasia is a hallmark of Crohn's disease (CD), and C reactive protein (CRP) is correlated with disease activity. The authors investigated whether mesenteric adipocytes may be a source of CRP in CD and whether inflammatory and bacterial triggers may stimulate its production by adipocytes.
Design: CRP expression in the mesenteric and subcutaneous fats of patients with CD and the correlation between CRP plasma concentrations and mesenteric messenger RNA (mRNA) levels were assessed. The impact of inflammatory and bacterial challenges on CRP synthesis was tested using an adipocyte cell line. Bacterial translocation to mesenteric fat was studied in experimental models of colitis and ileitis and in patients with CD.
Results: CRP expression was increased in the mesenteric fat of patients with CD, with mRNA levels being 80 ± 40 (p<0.05) and 140 ± 65 (p=0.04) times higher than in the mesenteric fat of patients with ulcerative colitis and in the subcutaneous fat of the same CD subjects, respectively, and correlated with plasma levels. Escherichia coli (1230 ± 175-fold, p<0.01), lipopolysaccharide (26 ± 0.5-fold, p<0.01), tumour necrosis factor α (15 ± 0.3-fold, p<0.01) and interleukin-6 (10 ± 0.7-fold, p<0.05) increased CRP mRNA levels in adipocyte 3T3-L1 cells. Bacterial translocation to mesenteric fat occurred in 13% and 27% of healthy and CD subjects, respectively, and was increased in experimental colitis and ileitis. Human mesenteric adipocytes constitutively expressed mRNA for TLR2, TLR4, NOD1 and NOD2.
Conclusion: Mesenteric fat is an important source of CRP in CD. CRP production by mesenteric adipocytes may be triggered by local inflammation and bacterial translocation to mesenteric fat, providing a mechanism whereby mesenteric fat hyperplasia may contribute to inflammatory response in CD.
Conflict of interest statement
Competing interests: None.
Figures
Figure 1
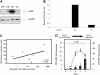
Overexpression of CRP mRNA and protein in mesenteric fat in CD. (A) Comparison of CRP expressions measured by real-time PCR in the mesenteric fat of patients with CD, patients with UC and control individuals. Significantly higher levels of CRP transcript levels were found in patients with CD compared to patients with UC and controls. Results are expressed as mean±SEM. (B) CRP mRNA levels in samples of mesenteric adipose tissue (Mes.) and subcutaneous fat (S/c) taken from the same patients with CD (n=6), patients with UC (n=3) or patients without IBD (n=3). Unlike in UC and control subjects in whom CRP mRNA levels are similar in mesenteric and subcutaneous fats, the CRP gene is overexpressed in mesenteric fat compared to subcutaneous fat in patients with CD. Median values are indicated by the horizontal line. (C) Expression of CRP gene in human mesenteric (Mes.) and subcutaneous (S/c) fat samples and in biopsy specimens of macroscopically and histologically healthy ileum (Ile.) or healthy colon (Col.) obtained from six UC, six CD and four control subjects using real-time PCR. Compared with adipose tissues, a modest (colon) or a weak (ileum) expression of CRP mRNA was observed in intestinal wall samples obtained from patients with UC, patients with CD or controls. Results are expressed as mean±SEM. CD, Crohn's disease; UC, ulcerative colitis; IBD, inflammatory bowel disease.
Figure 2
CRP expression by human mesenteric fat and correlation between mesenteric and plasma CRP concentrations in CD. (A) Levels of CRP by western blot analysis of mesenteric adipose tissue from one control patient who was operated on for colorectal cancer (Ctrl), CD subjects and UC subjects. Actin protein levels were determined to verify equal loading of samples. (B) The median CRP/β-actin ratio by western blot analysis is 0.01, 2.55 and 0.29 in controls (n=4), patients with CD (n=5) and patients with UC (n=4), respectively. (C) Correlation between plasma CRP concentrations measured at surgery and CRP mRNA levels quantified by real-time PCR in mesenteric adipose tissues taken during surgery from six patients with CD. (D) CRP mRNA levels parallel with and aP2 mRNA expression during the 9 days of 3T3-L1 pre-adipocyte differentiation into adipocytes on the indicated days. Results are expressed as fold induction as compared with undifferentiated pre-adipocytes (mean±SEM of three experiments). CD, Crohn's disease; UC, ulcerative colitis.
Figure 3
Induction of CRP expression in 3T3-L1 adipocytes following cytokine and/or bacterial challenge. (A) Differentiated 3T3-L1 adipocytes were stimulated with 10 ng/ml IL-6 or 50 ng/ml TNFα for 24 h. (B) Ability of 3T3-L1 pre-adipocytes and adipocytes to respond to the same bacterial challenge. Cells were infected with a strain of E coli isolated from a CD patient at a multiplicity of 100 pfu/cell for 1 h 30 min. Induction of CRP mRNA expression was then followed over time. (C) Differentiated 3T3-L1 adipocytes were infected with E coli or Lactobacillus spp. (infectivity ratio, 100 bacteria/cell) for 1 h 30 min, or treated with 100 ng/ml LPS, 1 μg/ml Pam3Cys or 10 μg/ml muramyl dipeptide for 24 h. (D) Synergistic effect of bacterial and cytokine stimuli on CRP expression in differentiated 3T3-L1 adipocytes. Cells were pre-treated with 50 ng/ml TNFα for 24 h before infection with E coli (infectivity ratio, 100 bacteria/cell). CRP mRNA expression was measured at 3 h after bacterial infection. In all experiments described in this figure, total RNA was isolated from 3T3-L1 cells at the indicated times, and levels of CRP mRNA were determined by real-time PCR. Results are expressed as the mean±SEM of three independent experiments and fold induction as compared with unstimulated adipocytes. IL, interleukin; LPS, lipopolysaccharide.
Figure 4
Bacterial translocation to mesenteric adipose tissue following acute DSS-induced colitis in mice, acute indomethacin-induced ileitis in rats and bacterial translocation in humans. (A) Rates of bacterial translocation (BT) to mesenteric adipose tissue (MAT) and mesenteric lymph nodes (MLN) in C57BL/6 mice with acute colitis induced by 2% DSS for 5 days and killed after 2 days on day 7 (n=8) and in control animals (n=20). (B) Rates of bacterial translocation (BT) to mesenteric adipose tissue (MAT) and mesenteric lymph nodes (MLN) in rats with acute ileitis induced by indomethacin (n=15) and in control animals (n=9). (C) Rates of bacterial translocation (BT) to mesenteric adipose tissue (MAT) and mesenteric lymph nodes (MLN) in patients without CD (controls and patients with UC, n=38) and patients with CD (n=22). UC, ulcerative colitis; DSS, dextran sulfate sodium.
Comment in
- Mesenteric fat in Crohn's disease: the hot spot of inflammation?
Siegmund B. Siegmund B. Gut. 2012 Jan;61(1):3-5. doi: 10.1136/gutjnl-2011-301354. Epub 2011 Nov 7. Gut. 2012. PMID: 22068165 No abstract available. - Visceral adipose tissue in Crohn's disease: Satan or Samaritan?
Subramanian V. Subramanian V. Inflamm Bowel Dis. 2012 Sep;18(9):1795-6. doi: 10.1002/ibd.22869. Epub 2012 Jan 24. Inflamm Bowel Dis. 2012. PMID: 22275295 No abstract available. - Mesenteric fat as a source of CRP and target for bacterial translocation in Crohn's disease.
Goode EC, Watson AJ. Goode EC, et al. Gastroenterology. 2012 Aug;143(2):496-8. doi: 10.1053/j.gastro.2012.06.018. Epub 2012 Jun 20. Gastroenterology. 2012. PMID: 22727663 No abstract available.
Similar articles
- Mesenteric fat in Crohn's disease: the hot spot of inflammation?
Siegmund B. Siegmund B. Gut. 2012 Jan;61(1):3-5. doi: 10.1136/gutjnl-2011-301354. Epub 2011 Nov 7. Gut. 2012. PMID: 22068165 No abstract available. - Differences in visceral fat and fat bacterial colonization between ulcerative colitis and Crohn's disease. An in vivo and in vitro study.
Zulian A, Cancello R, Ruocco C, Gentilini D, Di Blasio AM, Danelli P, Micheletto G, Cesana E, Invitti C. Zulian A, et al. PLoS One. 2013 Oct 24;8(10):e78495. doi: 10.1371/journal.pone.0078495. eCollection 2013. PLoS One. 2013. PMID: 24205244 Free PMC article. - Increased proinflammatory cytokines in mesenteric fat in major surgery and Crohn's disease.
Seifarth C, Hering NA, Arndt M, Lehmann KS, Stroux A, Weixler B, Kreis ME. Seifarth C, et al. Surgery. 2021 Jun;169(6):1328-1332. doi: 10.1016/j.surg.2020.11.039. Epub 2021 Jan 8. Surgery. 2021. PMID: 33431185 - Visceral adiposity and inflammatory bowel disease.
Rowan CR, McManus J, Boland K, O'Toole A. Rowan CR, et al. Int J Colorectal Dis. 2021 Nov;36(11):2305-2319. doi: 10.1007/s00384-021-03968-w. Epub 2021 Jun 9. Int J Colorectal Dis. 2021. PMID: 34104989 Review. - [Bacterial translocation in Crohn disease].
Laffineur G, Lescut D, Vincent P, Quandalle P, Wurtz A, Colombel JF. Laffineur G, et al. Gastroenterol Clin Biol. 1992;16(10):777-81. Gastroenterol Clin Biol. 1992. PMID: 1478405 Review. French.
Cited by
- Assessment of body composition-related imaging parameters indicative of sarcopenia in Chinese patients with Crohn's disease: correlation with disease severity and biologic efficacy.
Xie C, Qian W, Shang L, Wang X, Zhang J, Ma X, Pang X, Zhu L, Xi Q, Teng Y, Chen W. Xie C, et al. Am J Transl Res. 2024 Oct 15;16(10):5427-5440. doi: 10.62347/ZPZR8134. eCollection 2024. Am J Transl Res. 2024. PMID: 39544770 Free PMC article. - Comprehensive assessment of distinct abdominal fat compartments beyond liver content in overweight/obese patients using MRI and ultrasound imaging.
Chen Y, Zhang T, Qin B, Zhang R, Liu M, Guo R, Zhu Y, Zeng J, Chen Y. Chen Y, et al. Abdom Radiol (NY). 2024 Sep 21. doi: 10.1007/s00261-024-04591-3. Online ahead of print. Abdom Radiol (NY). 2024. PMID: 39305293 - A clinician's perspective on the new organ mesentery and non-vascular mesenteropathies.
Ahmed M. Ahmed M. Front Physiol. 2024 Sep 4;15:1336908. doi: 10.3389/fphys.2024.1336908. eCollection 2024. Front Physiol. 2024. PMID: 39296517 Free PMC article. Review. - Role of gut microbiota in Crohn's disease pathogenesis: Insights from fecal microbiota transplantation in mouse model.
Wu Q, Yuan LW, Yang LC, Zhang YW, Yao HC, Peng LX, Yao BJ, Jiang ZX. Wu Q, et al. World J Gastroenterol. 2024 Aug 21;30(31):3689-3704. doi: 10.3748/wjg.v30.i31.3689. World J Gastroenterol. 2024. PMID: 39193000 Free PMC article. - Microbiota-gut-brain axis: interplay between microbiota, barrier function and lymphatic system.
Zhuang M, Zhang X, Cai J. Zhuang M, et al. Gut Microbes. 2024 Jan-Dec;16(1):2387800. doi: 10.1080/19490976.2024.2387800. Epub 2024 Aug 25. Gut Microbes. 2024. PMID: 39182226 Free PMC article. Review.
References
- Black S, Kushner I, Samols D. C-reactive protein. J Biol Chem 2004;279:48487–90 - PubMed
- Bottazzi B, Doni A, Garlanda C, et al. An integrated view of humoral innate immunity: pentraxins as a paradigm. Annu Rev Immunol 2010;28:157–83 - PubMed
- Henriksen M, Jahnsen J, Lygren I, et al. C-reactive protein: a predictive factor and marker of inflammation in inflammatory bowel disease. Results from a prospective population-based study. Gut 2008;57:1518–23 - PubMed
- Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn's disease. N Engl J Med 2010;362:1383–95 - PubMed
- Treton X, Bouhnik Y, Mary JY, et al. Azathioprine withdrawal in patients with Crohn's disease maintained on prolonged remission: a high risk of relapse. Clin Gastroenterol Hepatol 2009;7:80–5 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous