Linkage of gut microbiome with cognition in hepatic encephalopathy - PubMed (original) (raw)
Linkage of gut microbiome with cognition in hepatic encephalopathy
Jasmohan S Bajaj et al. Am J Physiol Gastrointest Liver Physiol. 2012.
Abstract
Hepatic encephalopathy (HE) has been related to gut bacteria and inflammation in the setting of intestinal barrier dysfunction. We aimed to link the gut microbiome with cognition and inflammation in HE using a systems biology approach. Multitag pyrosequencing (MTPS) was performed on stool of cirrhotics and age-matched controls. Cirrhotics with/without HE underwent cognitive testing, inflammatory cytokines, and endotoxin analysis. Patients with HE were compared with those without HE using a correlation-network analysis. A select group of patients with HE (n = 7) on lactulose underwent stool MTPS before and after lactulose withdrawal over 14 days. Twenty-five patients [17 HE (all on lactulose, 6 also on rifaximin) and 8 without HE, age 56 ± 6 yr, model for end-stage liver disease score 16 ± 6] and ten controls were included. Fecal microbiota in cirrhotics were significantly different (higher Enterobacteriaceae, Alcaligeneceae, and Fusobacteriaceae and lower Ruminococcaceae and Lachnospiraceae) compared with controls. We found altered flora (higher Veillonellaceae), poor cognition, endotoxemia, and inflammation (IL-6, TNF-α, IL-2, and IL-13) in HE compared with cirrhotics without HE. In the cirrhosis group, Alcaligeneceae and Porphyromonadaceae were positively correlated with cognitive impairment. Fusobacteriaceae, Veillonellaceae, and Enterobacteriaceae were positively and Ruminococcaceae negatively related to inflammation. Network-analysis comparison showed robust correlations (all P < 1E-5) only in the HE group between the microbiome, cognition, and IL-23, IL-2, and IL-13. Lactulose withdrawal did not change the microbiome significantly beyond Fecalibacterium reduction. We concluded that cirrhosis, especially when complicated with HE, is associated with significant alterations in the stool microbiome compared with healthy individuals. Specific bacterial families (Alcaligeneceae, Porphyromonadaceae, Enterobacteriaceae) are strongly associated with cognition and inflammation in HE.
Figures
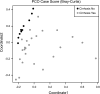
Fig. 1.
Principal coordinate analysis of the fecal microbiome of controls and cirrhotic patients. This graph shows the variation in fecal microbiome plotted on a principal coordinate analysis plot. Points that are closer to each other are similar with respect to their stool microbiota. The healthy controls represented by the black dots are clustered together, whereas the cirrhotic patients represented by the gray dots are distant from the controls. This indicates a difference in the stool microbiome of healthy controls compared to cirrhotic patients.
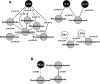
Fig. 2.
Correlation-network analysis of cirrhotic patients with and without hepatic encephalopathy (HE). Only correlations with a coefficient >r = 0.90 are displayed. Gray nodes indicate microbiome families; white nodes indicate cognitive tests; and black nodes are serum inflammatory markers. A black line connecting nodes indicates positive correlation, and a dashed line indicates negative correlation >0.90. The P values for the correlations are displayed on the lines connecting the nodes in A and B. MELD, model for end-stage liver disease score; DST, digit symbol test; LDTe, line drawing test errors. A: patients with HE (n = 17) have a high number of significant correlations. There are significant positive correlations between IL-23 and several bacterial families. Prevotellaceae and Fusobacteriaceae are positively correlated with inflammation. Because a low score on DST and high one on LDTe indicate poor performance, Alcaligenaceae and Porphyromonadaceae were correlated with poor cognition. The P values for all these correlations are less than the 4th decimal place, indicating a very high significance. B: patients without HE have very few significant correlations (n = 8). There was a significant negative correlation between MELD score and Ruminococcaceae and a positive correlation between Veillonellaceae and Porphyromonadaceae.
Similar articles
- Modulation of the metabiome by rifaximin in patients with cirrhosis and minimal hepatic encephalopathy.
Bajaj JS, Heuman DM, Sanyal AJ, Hylemon PB, Sterling RK, Stravitz RT, Fuchs M, Ridlon JM, Daita K, Monteith P, Noble NA, White MB, Fisher A, Sikaroodi M, Rangwala H, Gillevet PM. Bajaj JS, et al. PLoS One. 2013;8(4):e60042. doi: 10.1371/journal.pone.0060042. Epub 2013 Apr 2. PLoS One. 2013. PMID: 23565181 Free PMC article. Clinical Trial. - Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation.
Bajaj JS, Hylemon PB, Ridlon JM, Heuman DM, Daita K, White MB, Monteith P, Noble NA, Sikaroodi M, Gillevet PM. Bajaj JS, et al. Am J Physiol Gastrointest Liver Physiol. 2012 Sep 15;303(6):G675-85. doi: 10.1152/ajpgi.00152.2012. Epub 2012 Jul 19. Am J Physiol Gastrointest Liver Physiol. 2012. PMID: 22821944 Free PMC article. - A longitudinal systems biology analysis of lactulose withdrawal in hepatic encephalopathy.
Bajaj JS, Gillevet PM, Patel NR, Ahluwalia V, Ridlon JM, Kettenmann B, Schubert CM, Sikaroodi M, Heuman DM, Crossey MM, Bell DE, Hylemon PB, Fatouros PP, Taylor-Robinson SD. Bajaj JS, et al. Metab Brain Dis. 2012 Jun;27(2):205-15. doi: 10.1007/s11011-012-9303-0. Epub 2012 Apr 12. Metab Brain Dis. 2012. PMID: 22527995 - The role of microbiota in hepatic encephalopathy.
Bajaj JS. Bajaj JS. Gut Microbes. 2014 May-Jun;5(3):397-403. doi: 10.4161/gmic.28684. Epub 2014 Apr 1. Gut Microbes. 2014. PMID: 24690956 Free PMC article. Review. - Gut microbiota: its role in hepatic encephalopathy.
Rai R, Saraswat VA, Dhiman RK. Rai R, et al. J Clin Exp Hepatol. 2015 Mar;5(Suppl 1):S29-36. doi: 10.1016/j.jceh.2014.12.003. Epub 2014 Dec 16. J Clin Exp Hepatol. 2015. PMID: 26041954 Free PMC article. Review.
Cited by
- The microbiota in cirrhosis and its role in hepatic decompensation.
Trebicka J, Macnaughtan J, Schnabl B, Shawcross DL, Bajaj JS. Trebicka J, et al. J Hepatol. 2021 Jul;75 Suppl 1(Suppl 1):S67-S81. doi: 10.1016/j.jhep.2020.11.013. J Hepatol. 2021. PMID: 34039493 Free PMC article. Review. - Role of tenofovir dipivoxil in gut microbiota recovery from HBV-infection induced dysbiosis.
Long J, Saw M, Zhang P, Wang L, Li L, Ren H, Liu C, Ma Z, Zhang J, Wang B. Long J, et al. BMC Microbiol. 2024 Sep 20;24(1):359. doi: 10.1186/s12866-024-03457-4. BMC Microbiol. 2024. PMID: 39304810 Free PMC article. - Gut microbiota, inflammation and hepatic encephalopathy: a puzzle with a solution in sight.
Dhiman RK. Dhiman RK. J Clin Exp Hepatol. 2012 Sep;2(3):207-10. doi: 10.1016/j.jceh.2012.08.004. J Clin Exp Hepatol. 2012. PMID: 25755435 Free PMC article. No abstract available. - Gut dysbiosis in Huntington's disease: associations among gut microbiota, cognitive performance and clinical outcomes.
Wasser CI, Mercieca EC, Kong G, Hannan AJ, McKeown SJ, Glikmann-Johnston Y, Stout JC. Wasser CI, et al. Brain Commun. 2020 Jul 24;2(2):fcaa110. doi: 10.1093/braincomms/fcaa110. eCollection 2020. Brain Commun. 2020. PMID: 33005892 Free PMC article. - Effects of N-acetylcysteine on cytokines in non-acetaminophen acute liver failure: potential mechanism of improvement in transplant-free survival.
Stravitz RT, Sanyal AJ, Reisch J, Bajaj JS, Mirshahi F, Cheng J, Lee WM; Acute Liver Failure Study Group. Stravitz RT, et al. Liver Int. 2013 Oct;33(9):1324-31. doi: 10.1111/liv.12214. Epub 2013 Jun 19. Liver Int. 2013. PMID: 23782487 Free PMC article. Clinical Trial.
References
- Bajaj JS. Review article: the modern management of hepatic encephalopathy. Aliment Pharmacol Ther 31: 537– 547, 2010. . - PubMed
- Bajaj JS, Hafeezullah M, Franco J, Varma RR, Hoffmann RG, Knox JF, Hischke D, Hammeke TA, Pinkerton SD, Saeian K. Inhibitory control test for the diagnosis of minimal hepatic encephalopathy. Gastroenterology 135: 1591– 1600, 2008. . - PubMed
- Bajaj JS, Heuman DM, Wade JB, Gibson DP, Saeian K, Wegelin JA, Hafeezullah M, Bell DE, Sterling RK, Stravitz RT, Fuchs M, Luketic V, Sanyal AJ. Rifaximin improves driving simulator performance in a randomized trial of patients with minimal hepatic encephalopathy. Gastroenterology 140: 478– 487, 2011. . - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 AT004428/AT/NCCIH NIH HHS/United States
- R01AA020203/AA/NIAAA NIH HHS/United States
- R01 AA020203/AA/NIAAA NIH HHS/United States
- U01AT004428/AT/NCCIH NIH HHS/United States
- R01 AA020203-01/AA/NIAAA NIH HHS/United States
- R01 DK087913-01A1/DK/NIDDK NIH HHS/United States
- U01 AT004428-03/AT/NCCIH NIH HHS/United States
- R01 DK087913/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources