Blood-brain barrier breakdown in acute and chronic cerebrovascular disease - PubMed (original) (raw)
Review
Blood-brain barrier breakdown in acute and chronic cerebrovascular disease
Yi Yang et al. Stroke. 2011 Nov.
Abstract
Disruptions of the blood-brain barrier (BBB) and edema formation both play key roles in the development of neurological dysfunction in acute and chronic cerebral ischemia. Animal studies have revealed the molecular cascades that are initiated with hypoxia/ischemia in the cells forming the neurovascular unit and that contribute to cell death. Matrix metalloproteinases cause reversible degradation of tight junction proteins early after the onset of ischemia, and a delayed secondary opening during a neuroinflammatory response occurring from 24 to 72 hours after. Cyclooxygenases are important in the delayed opening as the neuroinflammatory response progresses. An early opening of the BBB within the 3-hour therapeutic window for tissue-type plasminogen activator can allow it to enter the brain and increase the risk of hemorrhage. Chronic hypoxic hypoperfusion opens the BBB, which contributes to the cognitive changes seen with lacunar strokes and white matter injury in subcortical ischemic vascular disease. This review will describe the molecular and cellular events associated with BBB disruption and potential therapies directed toward restoring the integrity of the neurovascular unit.
Figures
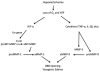
Fig. 1
Mechanism for MMP-mediated BBB disruption in hypoxia/ischemia. Induction of hypoxia inducible factor-α (HIF-α) by loss of oxygen and ATP leads to activation of MMP-2. The constitutive enzyme, proMT1-MMP, is activated by the convertase, Furin, and it activates proMMP-2. Secondary neuroinflammation with the formation of cytokines (TNF-α and IL-1β) and induction of MMP-9 and -3 occurs. ProMMP-9 is activated by MMP-3 and free radicals. Active MMPs degrade the basal lamina and tight junctions of endothelial cells, thereby opening the blood–brain barrier (BBB). Opening of the BBB leads to vasogenic edema.
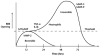
Fig. 2
Schematic drawing to show the theoretical mechanisms leading to the initial reversible opening of the BBB and the later more slowly irreversible opening. Reperfusion injury leads to a biphasic opening of the BBB. The early opening occurs several hours after the onset of reperfusion due to activation of the constitutive enzyme gelatinase A (MMP-2). This initial opening is transient and reversible. At 24 to 72 hours later, the inflammatory response leads to the induction of MMP-3 and MMP-9, which induce more intense and irreversible damage to the blood vessel.
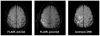
Fig. 3
Evidence of HARM for a representative acute stroke patient with early blood–brain barrier (BBB) disruption (woman, 81 years old, baseline NIHSS 17, no tPA). Left: FLAIR pre-Gd contrast, Middle: FLAIR post-Gd contrast, Left: isotropic DWI (b =1000). FLAIR enhancement was never seen before Gd contrast. After Gd contrast, FLAIR images were positive for HARM. (permission pending)
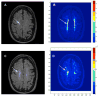
Fig. 4
Two subcortical ischemic vascular disease (SIVD) patients with white matter hyperintensities (WMHs). A) FLAIR MRI shows WMHs in the centrum semiovale (arrowhead) without involvement of the cortex. B) The corresponding permeability map has regions of moderately increased permeability (light blue) and high permeability (red). C) FLAIR image from another SIVD patient with larger white matter lesions (arrowhead). D) Permeability map shows increased permeability is limited to two small regions within the WMHs.
Similar articles
- Neurological diseases in relation to the blood-brain barrier.
Rosenberg GA. Rosenberg GA. J Cereb Blood Flow Metab. 2012 Jul;32(7):1139-51. doi: 10.1038/jcbfm.2011.197. Epub 2012 Jan 18. J Cereb Blood Flow Metab. 2012. PMID: 22252235 Free PMC article. Review. - Mechanisms in blood-brain barrier opening and metabolism-challenged cerebrovascular ischemia with emphasis on ischemic stroke.
Sarvari S, Moakedi F, Hone E, Simpkins JW, Ren X. Sarvari S, et al. Metab Brain Dis. 2020 Aug;35(6):851-868. doi: 10.1007/s11011-020-00573-8. Epub 2020 Apr 15. Metab Brain Dis. 2020. PMID: 32297170 Free PMC article. Review. - Vascular tight junction disruption and angiogenesis in spontaneously hypertensive rat with neuroinflammatory white matter injury.
Yang Y, Kimura-Ohba S, Thompson JF, Salayandia VM, Cossé M, Raz L, Jalal FY, Rosenberg GA. Yang Y, et al. Neurobiol Dis. 2018 Jun;114:95-110. doi: 10.1016/j.nbd.2018.02.012. Epub 2018 Feb 24. Neurobiol Dis. 2018. PMID: 29486300 Free PMC article. - Endothelial Adora2a Activation Promotes Blood-Brain Barrier Breakdown and Cognitive Impairment in Mice with Diet-Induced Insulin Resistance.
Yamamoto M, Guo DH, Hernandez CM, Stranahan AM. Yamamoto M, et al. J Neurosci. 2019 May 22;39(21):4179-4192. doi: 10.1523/JNEUROSCI.2506-18.2019. Epub 2019 Mar 18. J Neurosci. 2019. PMID: 30886019 Free PMC article. - Normobaric hyperoxia slows blood-brain barrier damage and expands the therapeutic time window for tissue-type plasminogen activator treatment in cerebral ischemia.
Liang J, Qi Z, Liu W, Wang P, Shi W, Dong W, Ji X, Luo Y, Liu KJ. Liang J, et al. Stroke. 2015 May;46(5):1344-1351. doi: 10.1161/STROKEAHA.114.008599. Epub 2015 Mar 24. Stroke. 2015. PMID: 25804925 Free PMC article.
Cited by
- Inflammation in neurological and psychiatric diseases.
Khansari PS, Sperlagh B. Khansari PS, et al. Inflammopharmacology. 2012 Jun;20(3):103-7. doi: 10.1007/s10787-012-0124-x. Epub 2012 Feb 24. Inflammopharmacology. 2012. PMID: 22361843 Review. - Interactions of SARS-CoV-2 with the Blood-Brain Barrier.
Erickson MA, Rhea EM, Knopp RC, Banks WA. Erickson MA, et al. Int J Mol Sci. 2021 Mar 6;22(5):2681. doi: 10.3390/ijms22052681. Int J Mol Sci. 2021. PMID: 33800954 Free PMC article. Review. - Potential roles of HDAC inhibitors in mitigating ischemia-induced brain damage and facilitating endogenous regeneration and recovery.
Fessler EB, Chibane FL, Wang Z, Chuang DM. Fessler EB, et al. Curr Pharm Des. 2013;19(28):5105-20. doi: 10.2174/1381612811319280009. Curr Pharm Des. 2013. PMID: 23448466 Free PMC article. Review. - TIMP1 preserves the blood-brain barrier through interacting with CD63/integrin β 1 complex and regulating downstream FAK/RhoA signaling.
Tang J, Kang Y, Huang L, Wu L, Peng Y. Tang J, et al. Acta Pharm Sin B. 2020 Jun;10(6):987-1003. doi: 10.1016/j.apsb.2020.02.015. Epub 2020 Mar 5. Acta Pharm Sin B. 2020. PMID: 32642407 Free PMC article. - Microglial activation and blood-brain barrier permeability in cerebral small vessel disease.
Walsh J, Tozer DJ, Sari H, Hong YT, Drazyk A, Williams G, Shah NJ, O'Brien JT, Aigbirhio FI, Rosenberg G, Fryer TD, Markus HS. Walsh J, et al. Brain. 2021 Jun 22;144(5):1361-1371. doi: 10.1093/brain/awab003. Brain. 2021. PMID: 34000009 Free PMC article.
References
- Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–1376. - PubMed
- Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. PharmacolRev. 2005;57:173–185. - PubMed
- Dore-Duffy P. Pericytes: Pluripotent cells of the blood brain barrier. Curr Pharm Des. 2008;14:1581–1593. - PubMed
Publication types
MeSH terms
Grants and funding
- R21NS066418/NS/NINDS NIH HHS/United States
- R01 NS052305/NS/NINDS NIH HHS/United States
- R01 NS045847/NS/NINDS NIH HHS/United States
- R21 NS066418/NS/NINDS NIH HHS/United States
- R21 NS066418-01/NS/NINDS NIH HHS/United States
- R01 NS052305-01/NS/NINDS NIH HHS/United States
- R01 NS021169-11/NS/NINDS NIH HHS/United States
- R01NS045847/NS/NINDS NIH HHS/United States
- R01 NS045847-01/NS/NINDS NIH HHS/United States
- R01NS052305/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources