BRCA1 is required for postreplication repair after UV-induced DNA damage - PubMed (original) (raw)
BRCA1 is required for postreplication repair after UV-induced DNA damage
Shailja Pathania et al. Mol Cell. 2011.
Abstract
BRCA1 contributes to the response to UV irradiation. Utilizing its BRCT motifs, it is recruited during S/G2 to UV-damaged sites in a DNA replication-dependent but nucleotide excision repair (NER)-independent manner. More specifically, at UV-stalled replication forks, it promotes photoproduct excision, suppression of translesion synthesis, and the localization and activation of replication factor C complex (RFC) subunits. The last function, in turn, triggers post-UV checkpoint activation and postreplicative repair. These BRCA1 functions differ from those required for DSBR.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
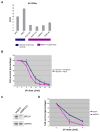
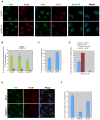
Figure 1. BRCA1−/− tumors and BRCA1- Depleted Cells are hypersensitive to UV-C
(A) Normal primary human breast epithelial cells (BPE/IMEC) and BRCA1−/− tumor cell lines were irradiated with increasing doses of UV-C and 7 days later assayed for colony formation. Error bars represent the standard deviation between two, independent experiments. (B) HCC1937, a BRCA1 mutant breast cancer cell line that is hypersensitive to UV, was reconstituted with BRCA1 cDNA or backbone vector. Reconstituted cells were irradiated and assayed for colony formation. Error bars represent the standard deviation in two independent experiments. (C) Western blot analysis of BRCA1 protein abundance in control (shGFP) and BRCA1- depleted (shBRCA1) U2OS cells. (D) These cells were also exposed to increasing doses of UV-C and assayed for colony formation.
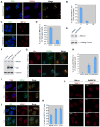
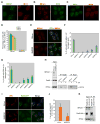
Figure 2. Recruitment of BRCA1 to sites of UV Damage
(A) Asynchronously growing U2OS cells were UV-irradiated (30J/m2) through micropore filters. 1hr post irradiation, cells were fixed and co-immunostained with CPD and BRCA1 Abs. (B) U2OS cells synchronized in S, G1, or in G0/G1 were UV- irradiated and fixed 1hr later for co-immunostaining with BRCA1 and CPD Abs. The error bars represent the standard deviation in three, separate experiments. (C) S phase- synchronized U2OS cells were incubated in the presence of aphidicolin (2μg/ml) for 1hr to block replication before UV irradiation (30J/m2). 2hrs later, the cells were co-immunostained for BRCA1 and CPD. (D) Quantization of BRCA1 localization to CPD positive sites in the experiment described in (C). Error bars represent the standard deviation in three, independent experiments. (E) BRCA1 abundance in control (DMSO) and aphidicolin- treated U2OS cells. (F) A myc- tagged, BRCA1 BRCT domain containing fragment (B1F6-myc) and a point-mutated derivative (B1F6-M1775R-myc) were each transfected into U2OS cells, and extracts were analyzed for expression of endogenous BRCA1 and the myc-tagged fragments. (G) B1F6-myc and B1F6-M1775R-myc transfected U2OS cells were UV- irradiated, and BRCA1 Ab (MS110 – directed against an N terminal epitope) was used to detect the recruitment of endogenous BRCA1 to the UV damage sites. Ab to myc was used to mark the cells expressing the myc tagged fragments. (H) Myc-positive cells were analyzed for endogenous BRCA1 localization at UV damage sites. Error bars represent standard deviation in three independent experiments. (I, J) U2OS cells were irradiated and immunostained for BRCA1, XPA and TFIIH. Cells were fixed at the indicated times and immunostained with the respective antibodies. (K) Localization of BRCA1 at CPD-rich UV damage sites was analyzed in two NER- deficient cell lines (XPA−/− and XPC−/−). A primary fibroblast strain, IMR90, was used as a WT control. All three cultures were UV- irradiated (30J/m2) and co-immunostained for CPD and BRCA1. The fraction of cells containing BRCA1 and CPD colocalized at UV damage spots is plotted. Error bars represent standard deviation in three independent experiments. (L) U2OS cells infected with control (shLuc) and ShBRCA1-encoding lentiviruses were irradiated with 20J/m2 and fixed at 1hr and 20hr after UV. Cells were immunostained with Ab to 53BP1.
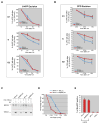
Figure 3. Excision of UV-induced lesions is BRCA1- dependent in S and G2 phase cells
(A, B) U2OS cells infected with control (shLuc) and shBRCA1-encoding lentiviruses were irradiated with 20J/m2 and 0.5J/m2 and the excision efficiencies of 6-4PP and CPD were analyzed, respectively, by FACS. Cells were gated for S, G2 and G1 phase to study damage excision during different cell cycle phases. (C) Western blot analysis of ERCC1 and BRCA1 in chromatin extract prepared from unirradiated and UV-irradiated HeLa cells infected with lentiviruses encoding a Luc (control) or a BRCA1 hairpin. Where indicated, cells were pre-treated with HU (1mM) for 16 hours to block replication and arrest cells at the G1/S boundary. Cells were harvested 4 hours after UV exposure (30J/m2). (D) GM04312 (XPA−/−) cells were infected, in parallel, with control (ShLuc) and BRCA1 shRNA- encoding lentiviruses, plated in triplicate, and irradiated with the indicated doses of UV. Colony formation was assayed after 7 days. Error bars represent the standard deviation in two independent experiments. (E) XPA−/−, XPC−/− and U2OS cells that were infected with shBRCA1 or shLuc lentiviruses, were arrested at G1/S by incubation with 1mM HU for 16hrs, , and then irradiated through micropore filters. Cells were fixed 30 minutes later and gap repair was analyzed by measuring in-situ DNA synthesis in presence bio-dUTP. The fraction of cells that revealed bio-dUTP staining in UV damage sites was plotted as a mean of three, separate experiments. Error bars represent the standard deviation between these experiments.
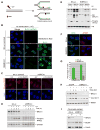
Figure 4. Generation of RPA-coated ssDNA after UV Damage is Largely BRCA1 Dependent
(A) Model. UV-induced damage in replicating cells generates stalled forks. (B) BrdU assay for detection of ssDNA after UV irradiation. U2OS cells were fixed 4 hours after irradiation and immunostained for BrdU with and without denaturation of DNA with HCl. (C) ShLuc- and shBRCA1- transduced U2OS cells were UV irradiated (5J/m2), fixed 4 hours later and immunostained with phospho- RPA32 ab. (D) ShLuc- and shBRCA1- infected U2OS cells were UV- irradiated (30J/m2) and collected at the indicated time points. The western blot was immunostained for phospho-RPA32, BRCA1 and GAPDH (loading control). (E) ShLuc- and shBRCA1- transduced U2OS cells were transfected with myc- tagged WT BRCA1 in parallel with a vector control. 48 hours later, cells were UV- irradiated (30J/m2) and harvested 4hrs later to prepare chromatin extracts, which were then blotted. The western blot was immunostained for phospho-RPA32, BRCA1 (using MS110 and anti-myc) and histone H3 (loading control). (F) Localization of ATRIP in locally UV irradiated cells. ShLuc- and shBRCA1- transduced U2OS cells were UV irradiated (30J/m2), fixed 2 hours later, and co-immunostained for CPD and ATRIP. (G) Data collected from experiments depicted in (C) and (F) are plotted. Error bars represent the standard deviations in three independent experiments. (H) Effect of BRCA1 depletion on Chk1S317 phosphorylation after UV. shRNA-transduced U2OS cells were harvested at the indicated times after UV exposure (30J/m2) and analyzed for Chk1 phosphorylation. (I) ShLuc- and shBRCA1- transduced U2OS cells were UV irradiated (30J/m2). Chromatin was prepared from cells harvested 4 hours post UV. The western blot was immunostained for BRCA1, MCM7, and Cdc45.
Figure 5. BRCA1 Plays a Role in the Recruitment of PCNA and DNA Polymerase δ to Sites of UV Damage
(A) U2OS cells, infected with shGFP (control)- and shBRCA1- encoding lentiviruses, were UV- irradiated at 30J/m2. Cells were fixed 3 hours later and analyzed by IF for PCNA and Pol δ recruitment to CPD- positive foci. Repair synthesis (via in situ bio-dUTP incorporation) was analyzed, in parallel, 3 hours after irradiation. (B) The percentage of cells with the indicated proteins or bio-dUTP localized at CPD- positive foci were plotted for shGFP- and shBRCA1- transduced U2OS cells. (C) Percentage of cells with RPA colocalized with CPD is plotted for siRNA- transfected U2OS cells. (D) ShGFP and shBRCA1- transduced U2OS cells were transfected with siGAPDH and siXPA. 48 hours after transfection, cells were UV-irradiated (30J/m2), fixed 3hrs later, and co-immunostained for Pol δ and CPD. Percentage of CPD stained cells that were also positive for Pol δ was plotted. Error bars represent the standard deviation in three, independent experiments. (E) ShLuc and ShBRCA1-transduced U2OS cells were UV irradiated (30J/m2), fixed 2hrs later and immunostained for Rad51 and CPD. (F) Percentage of cells with Pol δ at sites of UV damage in siRNA treated cells.
Figure 6. BRCA1 is Required to Direct RFC Complex Localization and Function at UV- stalled Forks
(A, B, C) U2OS cells were irradiated (30J/m2), fixed 3hrs later, and co-immunostained for (A) BRCA1 and RFC1 or (B) BRCA1 and RFC4 or (C) RFC1 and RFC5. (D) ShLuc and ShBRCA1-infected U2OS cells were irradiated (30J/m2), fixed 3hrs later and co-immunostained for CPD and RFC1 or RFC4 or RFC5. CPD- positive cells were analyzed for the recruitment of RFC1,4,or 5 respectively. (E) ShLuc and ShRFC2- infected cells were irradiated (30J/m2), fixed 1hr later and immunostained for BRCA1. (F, G) ShLuc, shRFC1, shRFC2, shRFC3, shRFC4 and shRFC5 – infected U2OS cells were analyzed for recruitment of Pol δ and RPA, respectively, to sites of UV damage (30J/m2) at 3hrs after irradiation. (H) Chromatin extracts from unirradiated and UV-irradiated (30J/m2) ShLuc and ShBRCA1- transduced U2OS cells were immunoprecipitated with Rad9- and Rad1- specific antibody. The western blots were immunoblotted for BRCA1, Rad9 and RFC4. (I) Rad9-GFP transfected shLuc- and shBRCA1-infected cells were irradiated (30J/m2), fixed 3hrs later, and immunostained for CPD and examined directly for Rad9-GFP. (J) The fraction of CPD positive cells that also were positive for Rad9-GFP from (I) were plotted. Error bars represent standard deviation between three, separate experiments. (K) Chromatin extracts from cells transfected with an HA-tagged Rad9- encoding vector or the vector backbone plasmid, were immunoprecipitated with HA antibody. The western blot was immunoblotted for BRCA1, Rad9 and RFC4.
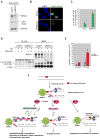
Figure 7. BRCA1 Plays a Role in Suppressing Translesional Synthesis
(A) Western blot analysis of the expression of GFP- tagged PolH in shLuc- and shBRCA1- infected U2OS cells. (B) Localization of eGFP-PolH in virus transduced U2OS cells irradiated with UV (30J/m2), fixed 4 hours later, and immunostained with GFP ab. (C) GFP positive cells with eGFP-PolH in the local UV irradiated spots, were counted. Percentages plotted are the mean of three independent experiments. (D) HA-tagged ubiquitin was expressed in U2OS cells transduced with shGFP and shBRCA1- encoding lentiviruses. Cells were irradiated (30J/m2), and harvested at the indicated time points. The whole cell extract (WCE) and the Anti-HA immunoprecipitates (IP:HA) were electrophoresed and blotted. The blot was reacted with BRCA1 and PCNA antibodies. (E) SupF assay. Unirradiated and UV- irradiated (500J/m2) SupF plasmid was transfected into shLuc and ShBRCA1-transduced 293T cells. Details of this assay are described in Supplemental Information. Data analyzed by counting blue (wildtype) and white (mutant) colonies from four, separate experiments is plotted (F) Model. BRCA1 function after UV-induced DNA damage.
Comment in
- BRCA1 forks over new roles in DNA-damage response- before and beyond the breaks.
Li L. Li L. Mol Cell. 2011 Oct 21;44(2):174-6. doi: 10.1016/j.molcel.2011.10.003. Mol Cell. 2011. PMID: 22017867 Free PMC article.
Similar articles
- Replication factor C recruits DNA polymerase delta to sites of nucleotide excision repair but is not required for PCNA recruitment.
Overmeer RM, Gourdin AM, Giglia-Mari A, Kool H, Houtsmuller AB, Siegal G, Fousteri MI, Mullenders LH, Vermeulen W. Overmeer RM, et al. Mol Cell Biol. 2010 Oct;30(20):4828-39. doi: 10.1128/MCB.00285-10. Epub 2010 Aug 16. Mol Cell Biol. 2010. PMID: 20713449 Free PMC article. - Gap-filling and bypass at the replication fork are both active mechanisms for tolerance of low-dose ultraviolet-induced DNA damage in the human genome.
Quinet A, Vessoni AT, Rocha CR, Gottifredi V, Biard D, Sarasin A, Menck CF, Stary A. Quinet A, et al. DNA Repair (Amst). 2014 Feb;14:27-38. doi: 10.1016/j.dnarep.2013.12.005. Epub 2013 Dec 28. DNA Repair (Amst). 2014. PMID: 24380689 - p53 prevents the accumulation of double-strand DNA breaks at stalled-replication forks induced by UV in human cells.
Squires S, Coates JA, Goldberg M, Toji LH, Jackson SP, Clarke DJ, Johnson RT. Squires S, et al. Cell Cycle. 2004 Dec;3(12):1543-57. doi: 10.4161/cc.3.12.1272. Epub 2004 Dec 28. Cell Cycle. 2004. PMID: 15539956 - Minding the gap: the underground functions of BRCA1 and BRCA2 at stalled replication forks.
Nagaraju G, Scully R. Nagaraju G, et al. DNA Repair (Amst). 2007 Jul 1;6(7):1018-31. doi: 10.1016/j.dnarep.2007.02.020. Epub 2007 Mar 26. DNA Repair (Amst). 2007. PMID: 17379580 Free PMC article. Review. - NER and DDR: classical music with new instruments.
Sertic S, Pizzi S, Lazzaro F, Plevani P, Muzi-Falconi M. Sertic S, et al. Cell Cycle. 2012 Feb 15;11(4):668-74. doi: 10.4161/cc.11.4.19117. Cell Cycle. 2012. PMID: 22373527 Review.
Cited by
- Replication stress and defective checkpoints make fallopian tube epithelial cells putative drivers of high-grade serous ovarian cancer.
Galhenage P, Zhou Y, Perry E, Loc B, Fietz K, Iyer S, Reinhardt F, Da Silva T, Botchkarev V, Chen J, Crum CP, Weinberg RA, Pathania S. Galhenage P, et al. Cell Rep. 2023 Oct 31;42(10):113144. doi: 10.1016/j.celrep.2023.113144. Epub 2023 Sep 19. Cell Rep. 2023. PMID: 37729060 Free PMC article. - BARD1 germline variants induce haploinsufficiency and DNA repair defects in neuroblastoma.
Randall MP, Egolf LE, Vaksman Z, Samanta M, Tsang M, Groff D, Evans JP, Rokita JL, Layeghifard M, Shlien A, Maris JM, Diskin SJ, Bosse KR. Randall MP, et al. J Natl Cancer Inst. 2024 Jan 10;116(1):138-148. doi: 10.1093/jnci/djad182. J Natl Cancer Inst. 2024. PMID: 37688570 Free PMC article. - RFWD3 promotes ZRANB3 recruitment to regulate the remodeling of stalled replication forks.
Moore CE, Yalcindag SE, Czeladko H, Ravindranathan R, Wijesekara Hanthi Y, Levy JC, Sannino V, Schindler D, Ciccia A, Costanzo V, Elia AEH. Moore CE, et al. J Cell Biol. 2023 May 1;222(5):e202106022. doi: 10.1083/jcb.202106022. Epub 2023 Apr 10. J Cell Biol. 2023. PMID: 37036693 Free PMC article. - Targeting the BRCA1/2 deficient cancer with PARP inhibitors: Clinical outcomes and mechanistic insights.
Ragupathi A, Singh M, Perez AM, Zhang D. Ragupathi A, et al. Front Cell Dev Biol. 2023 Mar 22;11:1133472. doi: 10.3389/fcell.2023.1133472. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37035242 Free PMC article. Review. - BARD1 germline variants induce haploinsufficiency and DNA repair defects in neuroblastoma.
Randall MP, Egolf LE, Vaksman Z, Samanta M, Tsang M, Groff D, Evans JP, Rokita JL, Layeghifard M, Shlien A, Maris JM, Diskin SJ, Bosse KR. Randall MP, et al. bioRxiv [Preprint]. 2023 Feb 3:2023.01.31.525066. doi: 10.1101/2023.01.31.525066. bioRxiv. 2023. PMID: 36778420 Free PMC article. Updated. Preprint.
References
- Al-Minawi AZ, Lee YF, Håkansson D, Johansson F, Lundin C, Saleh-Gohari N, Schultz N, Jenssen D, Bryant HE, Meuth M, et al. The ERCC1/XPF endonuclease is required for completion of homologous recombination at DNA replication forks stalled by inter-strand cross-links. Nucleic Acids Res. 2009;37:6400–6413. - PMC - PubMed
- Au WWY, Henderson BR. The BRCA1 RING and BRCT domains cooperate in targeting BRCA1 to ionizing radiation-induced nuclear foci. J Biol Chem. 2005;280:6993–7001. - PubMed
- Bartkova J, Horejsí Z, Koed K, Krämer A, Tort F, Zieger K, Guldberg P, Sehested M, Nesland JM, Lukas C, et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864–870. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50HG004233/HG/NHGRI NIH HHS/United States
- R01 CA095175/CA/NCI NIH HHS/United States
- R01 CA103920-05/CA/NCI NIH HHS/United States
- R01 GM073894/GM/NIGMS NIH HHS/United States
- T32 GM007753/GM/NIGMS NIH HHS/United States
- RC1 CA145867/CA/NCI NIH HHS/United States
- R21 CA144017/CA/NCI NIH HHS/United States
- P50 HG004233/HG/NHGRI NIH HHS/United States
- R01 CA103920/CA/NCI NIH HHS/United States
- P01NS047572/NS/NINDS NIH HHS/United States
- P30 AI060354/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous