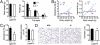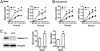Ablation of a galectin preferentially expressed in adipocytes increases lipolysis, reduces adiposity, and improves insulin sensitivity in mice - PubMed (original) (raw)
Ablation of a galectin preferentially expressed in adipocytes increases lipolysis, reduces adiposity, and improves insulin sensitivity in mice
Ri-Yao Yang et al. Proc Natl Acad Sci U S A. 2011.
Abstract
The breakdown of triglycerides, or lipolysis, is a tightly controlled process that regulates fat mobilization in accord with an animal's energy needs. It is well established that lipolysis is stimulated by hormones that signal energy demand and is suppressed by the antilipolytic hormone insulin. However, much still remains to be learned about regulation of lipolysis by intracellular signaling pathways in adipocytes. Here we show that galectin-12, a member of a β-galactoside-binding lectin family preferentially expressed by adipocytes, functions as an intrinsic negative regulator of lipolysis. Galectin-12 is primarily localized on lipid droplets and regulates lipolytic protein kinase A signaling by acting upstream of phosphodiesterase activity to control cAMP levels. Ablation of galectin-12 in mice results in increased adipocyte mitochondrial respiration, reduced adiposity, and ameliorated insulin resistance/glucose intolerance. This study identifies unique properties of this intracellular galectin that is localized to an organelle and performs a critical function in lipid metabolism. These findings add to the significant functions exhibited by intracellular galectins, and have important therapeutic implications for human metabolic disorders.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Galectin-12 deficiency reduces adiposity in mice. (A) Comparison of weights of visceral (epididymal) and subcutaneous (inguinal) white adipose tissue in Lgals12+/+ and _Lgals12_−/− mice (+/+, n = 13; −/−, n = 18), as well as interscapular brown adipose tissue (BAT) and body weight. (B) Linear regression analyses show that body weight and fat depot weight are highly correlated in Lgals12+/+ mice (epididymal, _R_2 = 0.586, P = 0.002; inguinal, _R_2 = 0.887, P < 0.0001), but not in _Lgals12_−/− mice (epididymal, _R_2 < 0.00001, _P_ = 0.992; inguinal, _R_2 = 0.015, _P_ = 0.676). (_C_) Triglyceride contents and adipocyte numbers of epidydymal fat depots from _Lgals12_+/+ (_n_ = 5) and _Lgals12_−/− (_n_ = 4) mice. (_D_) H&E staining of paraffin sections of epididymal fat depots from _Lgals12_+/+ and _Lgals12_−/− mice (representative of four experiments). Average diameters of >200 isolated adipocytes were determined from their digital images with ImageJ software using 100-μm Polybead polystyrene microspheres (Polysciences) as references. Results are from 22- to 24-wk-old males. Asterisks denote statistical significance (*P < 0.05).
Fig. 2.
Galectin-12 is a lipid droplet protein. (A) 3T3-L1 adipocytes were incubated for 2 h in serum-free DMEM. Protein levels of extracellular (in conditioned medium) and intracellular (cell-associated) galectin-12 and adiponectin were determined by Western blotting using specific antibodies. (B) Lipid droplets (LD) were purified from 3T3-L1 adipocytes by density gradient centrifugation, and the remaining cellular components were separated by differential centrifugation into five fractions containing plasma membrane (PM), high-density microsomes (HDM), low-density microsomes (LDM), cytosol, and mitochondria/nuclei (M/N). Levels of galectin-12, Glut-4, and perilipin A were determined in each fraction by Western blotting with respective specific antibodies. Each lane represents samples from an equal number of cells. (C) Immunostaining of 3T3 adipocytes showing galectin-12 (red) and perilipin A (green, Lower) on lipid droplets (green, Upper). (D) 3T3-L1 cells were incubated in the presence (MDI+) or absence (MDI−) of the adipogenic hormone mixture to induce adipocyte differentiation. At indicated time points, cells were extracted for analysis of galectin-12 expression by Western blotting. (E) 3T3-L1 cells at various periods during differentiation were stained for galectin-12 (red), lipid droplets (green), and the nuclei (blue). Data are representative of three experiments with similar results.
Fig. 3.
Galectin-12 deficiency results in elevated lipolysis in adipocytes. (A and B) Adipocytes were isolated from epididymal fat depots of Lgals12+/+ and _Lgals12_−/− mice (n = 3–6) on a regular diet ad libitum. Equal numbers of cells were incubated in the absence (A) or presence (B) of 0.1 μM of isoproterenol. Glycerol and nonesterified fatty acid (NEFA) released into the medium were measured at different time points of incubation at 37 °C. (C) siRNA-mediated knockdown was performed by electroporating 3T3-L1 adipocytes with either control siRNA (Ctrl) or a combination of two galectin-12–specific siRNAs (si12). Three days later, galectin-12 levels were analyzed by Western blotting, and lipolysis was determined by monitoring the release of fatty acids and glycerol 1.5 h after isopreterenol stimulation. Asterisks denote statistical significance (*P < 0.05). Similar results were observed in four (A and B) or three (C) experiments.
Fig. 4.
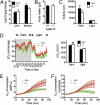
Tissue lipid content, total ambulatory activity, energy expenditure, and food intake in Lgals12+/+ and _Lgals12_−/− mice. (A) Lipid contents of liver and muscle were determined for Lgals12+/+ and _Lgals12_−/− male mice (n = 5 for each genotype), by the ethanolic-KOH saponification method. (B) Food intake of Lgals12+/+ (n = 5) and _Lgals12_−/− (n = 6). (C and D) Total ambulatory activity (distance of movement) and oxygen consumption rates (VO2) were determined by indirect calorimetry during the dark (7:00 PM to 7:00 AM) and light (7:00 AM to 7:00 PM) periods in Lgals12+/+ and _Lgals12_−/− male mice (n = 5 for each genotype) on standard diet. (E and F) Basal (E) or isoproterenol-stimulated (F) oxygen consumption of isolated epididymal white adipocytes from Lgals12+/+ and _Lgals12_−/− mice (n = 5–7) measured with the BD Oxygen Biosensor System. Oxygen consumption in E is expressed as normalized relative fluorescence unit (NRFU). Results in F are the ratios of oxygen consumption by adipocytes stimulated with isoproterenol to that by adipocytes under basal conditions. All animals were studied at 22 to 26 wk of age. Asterisks denote statistical significance (*P < 0.05).
Fig. 5.
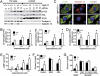
Galectin-12 ablation promotes PKA phosphorylation of HSL and association of phosphorylated HSL and ATGL with lipid droplets as a result of elevated cAMP levels. (A) Adipocytes from the epididymal fat depots of Lgals12+/+ and Lgals12−/− mice were incubated with indicated concentrations of isoproterenol before being separated into cytosol and fat cake. Lipolytic proteins in each fraction were analyzed by Western blotting with indicated antibodies. (B) Quantification of lipid droplet (LD)-associated p-HSL and HSL by densitometry of Western blots (n = 3 for each genotype). (C) Immunofluorescence of adipocytes differentiated from MEFs shows elevated levels of phospho-HSL associated with lipid droplets in Lgals12−/− adipocytes 15 min after treatment with 0.1 μM isoproterenol. (D) Quantification of lipid droplet-associated ATGL by densitometry of Western blots (n = 3 for each genotype). (E) Adipocytes from Lgals12+/+ (n = 3) and Lgals12−/− (n = 3) mice were incubated with indicated concentrations of isoproterenol for 5 min at 37 °C and intracellular cAMP levels were determined by ELISA. (F) Adipocytes from Lgals12+/+ and Lgals12−/− mice (n = 3–6) were treated with or without 0.1 mM IBMX or 1 U/mL ADA for 1 h at 37 °C and lipolysis was determined by measuring glycerol release. (G) cAMP/dbcAMP-stimulated lipolysis in adipocytes from Lgals12+/+ and Lgals12−/− mice (n = 4). Asterisks denote statistical significance (*P < 0.05). Results are representative of three experiments.
Fig. 6.
Galectin-12 deficiency reduces insulin resistance and glucose intolerance associated with weight gain. (A and B) AUC was computed from the plot of blood glucose levels as a function of time after intraperitoneal injection of Lgals12+/+ and _Lgals12_−/− mice with insulin (A) or glucose (B). The AUC values, which reflect insulin resistance (A) or glucose intolerance (B), were then plotted as a function of body weight. Note that insulin resistance and glucose intolerance correlate with body weight in _Lgals_12+/+ mice (insulin resistance vs. body weight, _R_2 = 0.583, P = 0.002; glucose intolerance vs. body weight, _R_2 = 0.353, P = 0.032). Such correlation was absent in _Lgals12_−/− mice (insulin resistance vs. body weight, _R_2 < 0.001, _P_ = 0.933; glucose intolerance vs. body weight, _R_2 = 0.004, _P_ = 0.829). (_C_) Changes in plasma insulin levels in mice weighing > 30 g during the first hour of the glucose tolerance test. (D and E) Plots of insulin resistance and glucose intolerance, as described in A and B, respectively, as a function of adiposity. Asterisks denote statistical significance (*P < 0.05). Results are representative of three to four experiments.
Comment in
- Burn control, an adipocyte-specific function for galectin-12.
Baum LG. Baum LG. Proc Natl Acad Sci U S A. 2011 Nov 15;108(46):18575-6. doi: 10.1073/pnas.1115738108. Epub 2011 Nov 4. Proc Natl Acad Sci U S A. 2011. PMID: 22058227 Free PMC article. No abstract available.
Similar articles
- Galectin-12: A protein associated with lipid droplets that regulates lipid metabolism and energy balance.
Yang RY, Havel PJ, Liu FT. Yang RY, et al. Adipocyte. 2012 Apr 1;1(2):96-100. doi: 10.4161/adip.19465. Adipocyte. 2012. PMID: 23700518 Free PMC article. - Burn control, an adipocyte-specific function for galectin-12.
Baum LG. Baum LG. Proc Natl Acad Sci U S A. 2011 Nov 15;108(46):18575-6. doi: 10.1073/pnas.1115738108. Epub 2011 Nov 4. Proc Natl Acad Sci U S A. 2011. PMID: 22058227 Free PMC article. No abstract available. - ABHD15 regulates adipose tissue lipolysis and hepatic lipid accumulation.
Stöckli J, Zadoorian A, Cooke KC, Deshpande V, Yau B, Herrmann G, Kebede MA, Humphrey SJ, James DE. Stöckli J, et al. Mol Metab. 2019 Jul;25:83-94. doi: 10.1016/j.molmet.2019.05.002. Epub 2019 May 6. Mol Metab. 2019. PMID: 31105056 Free PMC article. - The central role of perilipin a in lipid metabolism and adipocyte lipolysis.
Tansey JT, Sztalryd C, Hlavin EM, Kimmel AR, Londos C. Tansey JT, et al. IUBMB Life. 2004 Jul;56(7):379-85. doi: 10.1080/15216540400009968. IUBMB Life. 2004. PMID: 15545214 Review. - Regulation of adipocyte lipolysis.
Frühbeck G, Méndez-Giménez L, Fernández-Formoso JA, Fernández S, Rodríguez A. Frühbeck G, et al. Nutr Res Rev. 2014 Jun;27(1):63-93. doi: 10.1017/S095442241400002X. Epub 2014 May 28. Nutr Res Rev. 2014. PMID: 24872083 Review.
Cited by
- Targeted inhibition of galectin 1 by thiodigalactoside dramatically reduces body weight gain in diet-induced obese rats.
Mukherjee R, Kim SW, Park T, Choi MS, Yun JW. Mukherjee R, et al. Int J Obes (Lond). 2015 Sep;39(9):1349-58. doi: 10.1038/ijo.2015.74. Epub 2015 Apr 29. Int J Obes (Lond). 2015. PMID: 25920776 - Galectins: An Ancient Family of Carbohydrate Binding Proteins with Modern Functions.
Verkerke H, Dias-Baruffi M, Cummings RD, Arthur CM, Stowell SR. Verkerke H, et al. Methods Mol Biol. 2022;2442:1-40. doi: 10.1007/978-1-0716-2055-7_1. Methods Mol Biol. 2022. PMID: 35320517 Review. - Epigallocatechin gallate decreases plasma triglyceride, blood pressure, and serum kisspeptin in obese human subjects.
Chatree S, Sitticharoon C, Maikaew P, Pongwattanapakin K, Keadkraichaiwat I, Churintaraphan M, Sripong C, Sririwichitchai R, Tapechum S. Chatree S, et al. Exp Biol Med (Maywood). 2021 Jan;246(2):163-176. doi: 10.1177/1535370220962708. Epub 2020 Oct 12. Exp Biol Med (Maywood). 2021. PMID: 33045853 Free PMC article. Clinical Trial. - Yeast as a Model to Find New Drugs and Drug Targets for _VPS13_-Dependent Neurodegenerative Diseases.
Kaminska J, Soczewka P, Rzepnikowska W, Zoladek T. Kaminska J, et al. Int J Mol Sci. 2022 May 4;23(9):5106. doi: 10.3390/ijms23095106. Int J Mol Sci. 2022. PMID: 35563497 Free PMC article. Review. - Bisected, complex N-glycans and galectins in mouse mammary tumor progression and human breast cancer.
Miwa HE, Koba WR, Fine EJ, Giricz O, Kenny PA, Stanley P. Miwa HE, et al. Glycobiology. 2013 Dec;23(12):1477-90. doi: 10.1093/glycob/cwt075. Epub 2013 Sep 13. Glycobiology. 2013. PMID: 24037315 Free PMC article.
References
- Cummings RD, Liu F. In: Essentials of Glycobiology. Varki A, et al., editors. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 2009. pp. 475–488. - PubMed
- Wang JL, Gray RM, Haudek KC, Patterson RJ. Nucleocytoplasmic lectins. Biochim Biophys Acta. 2004;1673:75–93. - PubMed
- Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nat Rev Cancer. 2005;5:29–41. - PubMed
- Yang RY, Rabinovich GA, Liu FT. Galectins: Structure, function and therapeutic potential. Expert Rev Mol Med. 2008;10:e17. - PubMed
- Nakahara S, Oka N, Raz A. On the role of galectin-3 in cancer apoptosis. Apoptosis. 2005;10:267–275. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA093373/CA/NCI NIH HHS/United States
- U24 DK092993/DK/NIDDK NIH HHS/United States
- R01 AI020958/AI/NIAID NIH HHS/United States
- F32 HL009333/HL/NHLBI NIH HHS/United States
- RC1 DK087307/DK/NIDDK NIH HHS/United States
- R01 AR56343/AR/NIAMS NIH HHS/United States
- R01 HL09333/HL/NHLBI NIH HHS/United States
- AT002993/AT/NCCIH NIH HHS/United States
- R21 AT002993/AT/NCCIH NIH HHS/United States
- R01 AR056343/AR/NIAMS NIH HHS/United States
- R01 HL075675/HL/NHLBI NIH HHS/United States
- DK087307/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases