Mutant huntingtin's interaction with mitochondrial protein Drp1 impairs mitochondrial biogenesis and causes defective axonal transport and synaptic degeneration in Huntington's disease - PubMed (original) (raw)
. 2012 Jan 15;21(2):406-20.
doi: 10.1093/hmg/ddr475. Epub 2011 Oct 13.
Affiliations
- PMID: 21997870
- PMCID: PMC3276281
- DOI: 10.1093/hmg/ddr475
Mutant huntingtin's interaction with mitochondrial protein Drp1 impairs mitochondrial biogenesis and causes defective axonal transport and synaptic degeneration in Huntington's disease
Ulziibat P Shirendeb et al. Hum Mol Genet. 2012.
Abstract
The purpose of this study was to investigate the link between mutant huntingtin (Htt) and neuronal damage in relation to mitochondria in Huntington's disease (HD). In an earlier study, we determined the relationship between mutant Htt and mitochondrial dynamics/synaptic viability in HD patients. We found mitochondrial loss, abnormal mitochondrial dynamics and mutant Htt association with mitochondria in HD patients. In the current study, we sought to expand on our previous findings and further elucidate the relationship between mutant Htt and mitochondrial and synaptic deficiencies. We hypothesized that mutant Htt, in association with mitochondria, alters mitochondrial dynamics, leading to mitochondrial fragmentation and defective axonal transport of mitochondria in HD neurons. In this study, using postmortem HD brains and primary neurons from transgenic BACHD mice, we identified mutant Htt interaction with the mitochondrial protein Drp1 and factors that cause abnormal mitochondrial dynamics, including GTPase Drp1 enzymatic activity. Further, using primary neurons from BACHD mice, for the first time, we studied axonal transport of mitochondria and synaptic degeneration. We also investigated the effect of mutant Htt aggregates and oligomers in synaptic and mitochondrial deficiencies in postmortem HD brains and primary neurons from BACHD mice. We found that mutant Htt interacts with Drp1, elevates GTPase Drp1 enzymatic activity, increases abnormal mitochondrial dynamics and results in defective anterograde mitochondrial movement and synaptic deficiencies. These observations support our hypothesis and provide data that can be utilized to develop therapeutic targets that are capable of inhibiting mutant Htt interaction with Drp1, decreasing mitochondrial fragmentation, enhancing axonal transport of mitochondria and protecting synapses from toxic insults caused by mutant Htt.
© The Author 2011. Published by Oxford University Press. All rights reserved.
Figures
Figure 1.
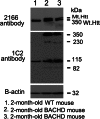
Identification of Htt in BACHD mice. Mutant Htt proteins were identified in cortical tissues from 2-month-old BACHD mice and control mice using two different Htt antibodies: MAB2166 (which recognizes both WT and mutant Htt) and 1C2 (which identifies expanded polyQ proteins). As shown, both full-length WT and mutant Htt proteins were found in the 2-month-old BACHD mice (lanes 2 and 3), and only full-length WT Htt was observed in the WT mice (lane 1). Western blot analysis using the 1C2 antibody (in the middle panel) showed that the BACHD mice cortex expressed four distinct bands: <350 kDa full-length mutant Htt, and 230, 115 and 82 kDa cleaved products of mutant Htt. A 115 kDa protein was identified in the WT mice. Bottom panel shows β-actin for equal loading.
Figure 2.
IP analysis in HD patients using the Drp1 antibody. (A) IP and immunoblotting analyses of Drp1 in cortical tissues from control subjects (lanes 1 and 2) and HD patients (lanes 3 and 4). (B) Immunoblotting analysis of Drp1 in control subjects (lanes 5 and 6) and HD patients (7 and 8). The Drp1 antibody recognizes the 82 kDa protein.
Figure 3.
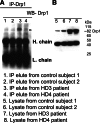
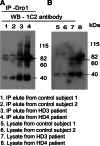
Co-IP analysis of Drp1 and mutant Htt antibodies in HD patients and control subjects. (A) IP with the Drp1antibody and immunoblotting with the mutant Htt-specific 1C2 antibody in control subjects (lanes 1 and 2) and HD patients (3 and 4). (B) Immunoblotting with the 1C2 antibody using protein lysates from control subjects (lanes 5 and 6) and HD patients (lanes 7 and 8). Mutant Htt-specific 1C2 antibody immunoreacted with two proteins: one with 82 kDa and the other with 40 kDa in IP elutes from HD patients.
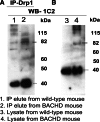
Figure 4.
IP analysis in BACHD mice using the Drp1 antibody. (A) IP and immunoblotting analyses of Drp1 in cortical tissues from WT mice (lane 1) and BACHD mice (lane 2). (B) Immunoblotting analysis of Drp1 in WT mice (lane 3) and BACHD mice (lane 4).
Figure 5.
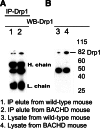
Co-IP analysis of Drp1 and mutant Htt antibodies in WT mice and BACHD mice. (A) IP with the Drp1 antibody and immunoblotting with the mutant Htt-specific 1C2 antibody in WT mice (lane 1) and BACHD mice (lane 2). (B) Immunoblotting with the 1C2 antibody using protein lysates from WT mice (lane 1) and BACHD mice (lane 2). The mutant Htt-specific 1C2 antibody immunoreacted with two proteins: one with 82 kDa and the other with 40 kDa in IP elutes from the BACHD mice.
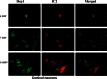
Figure 6.
Time-course, double-labeling analysis of Drp1 and mutant Htt in cortical primary neurons from BACHD mice. Drp1 (green) and mutant Htt (1C2 in red) accumulate in the cell neurites and soma over time. Drp1 co-localization increased with mutant Htt in DIV 14.
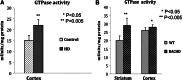
Figure 7.
Increased GTPase Drp1 enzymatic activity in HD patients and BACHD mice. (A) Significantly increased GTPase Drp1 enzymatic activity in the cortex of HD patients relative to control subjects. (B) Significantly increased Drp1 activity in the striatum and cortex of 2-month-old BACHD mice relative to non-transgenic littermates.
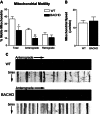
Figure 8.
Primary neurons from BACHD mice show reduced motile mitochondria and also anterograde axonal transport of mitochondria. (A) Percent of motile mitochondria in neuronal cultures of BACHD and WT mice. (B) Mitochondrial speed in BACHD and WT neurons. (C) Kymographs show anterograde movement of mitochondria. *P < 0.02; **P < 0.0005.
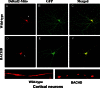
Figure 9.
Mitochondria are more fragmented in primary cortical neurons from BACHD mice. Upper panel shows DsRed-mito (A) and GFP (B) transfected (C, merged) cortical neurons from WT mice, and lower panel shows DsRed-mito (D) and GFP (E) transfected (F, merged) cortical neurons from BACHD mice.
Figure 10.
Mitochondrial fragmentation is prevented in neurons transfected with the Drp1 dominant negative mutation K38A. (A) Hippocampal primary neuron showing mitochondrial fragmentation. (B) Primary neurons transfected with WT Drp1 showing increased fragmentation. (C) Elongated mitochondria in WT neurons transfected with the Drp1.K38A mutation.
Figure 11.
Mutant Htt aggregates co-localize with oligomers in BACHD primary neurons. (A) Immunoreactivity mutant Htt oligomers (immunolabeled with the A11 antibody). (B) Mutant Htt. (C) Co-localization of mutant aggregates and oligomers.
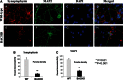
Figure 12.
Double-labeling analysis of synaptophysin and MAP2 in BACHD and WT primary neurons. Upper panel shows immunoreactivity of synaptophysin (A), MAP2 (B), DAPI (C) and merged (D) in WT neurons. Lower panel shows immunoreactivity of synaptophysin (E), MAP2 (F), DAPI (G) and merged (H) in WT neurons. Images are taken with a confocal microscope using a 60× objective. (B) Quantification of synaptophysin in BACHD and WT neurons. Significantly decreased synaptophysin was found in BACHD neurons.
Similar articles
- Mutant huntingtin, abnormal mitochondrial dynamics, defective axonal transport of mitochondria, and selective synaptic degeneration in Huntington's disease.
Reddy PH, Shirendeb UP. Reddy PH, et al. Biochim Biophys Acta. 2012 Feb;1822(2):101-10. doi: 10.1016/j.bbadis.2011.10.016. Epub 2011 Nov 4. Biochim Biophys Acta. 2012. PMID: 22080977 Free PMC article. Review. - S-nitrosylation of dynamin-related protein 1 mediates mutant huntingtin-induced mitochondrial fragmentation and neuronal injury in Huntington's disease.
Haun F, Nakamura T, Shiu AD, Cho DH, Tsunemi T, Holland EA, La Spada AR, Lipton SA. Haun F, et al. Antioxid Redox Signal. 2013 Oct 10;19(11):1173-84. doi: 10.1089/ars.2012.4928. Epub 2013 Jun 20. Antioxid Redox Signal. 2013. PMID: 23641925 Free PMC article. - Mutant huntingtin binds the mitochondrial fission GTPase dynamin-related protein-1 and increases its enzymatic activity.
Song W, Chen J, Petrilli A, Liot G, Klinglmayr E, Zhou Y, Poquiz P, Tjong J, Pouladi MA, Hayden MR, Masliah E, Ellisman M, Rouiller I, Schwarzenbacher R, Bossy B, Perkins G, Bossy-Wetzel E. Song W, et al. Nat Med. 2011 Mar;17(3):377-82. doi: 10.1038/nm.2313. Epub 2011 Feb 20. Nat Med. 2011. PMID: 21336284 Free PMC article. - Abnormal mitochondrial dynamics, mitochondrial loss and mutant huntingtin oligomers in Huntington's disease: implications for selective neuronal damage.
Shirendeb U, Reddy AP, Manczak M, Calkins MJ, Mao P, Tagle DA, Reddy PH. Shirendeb U, et al. Hum Mol Genet. 2011 Apr 1;20(7):1438-55. doi: 10.1093/hmg/ddr024. Epub 2011 Jan 21. Hum Mol Genet. 2011. PMID: 21257639 Free PMC article. - Mitochondrial Abnormalities and Synaptic Damage in Huntington's Disease: a Focus on Defective Mitophagy and Mitochondria-Targeted Therapeutics.
Sawant N, Morton H, Kshirsagar S, Reddy AP, Reddy PH. Sawant N, et al. Mol Neurobiol. 2021 Dec;58(12):6350-6377. doi: 10.1007/s12035-021-02556-x. Epub 2021 Sep 14. Mol Neurobiol. 2021. PMID: 34519969 Review.
Cited by
- Role of Mitochondrial Dysfunctions in Neurodegenerative Disorders: Advances in Mitochondrial Biology.
Kathiresan DS, Balasubramani R, Marudhachalam K, Jaiswal P, Ramesh N, Sureshbabu SG, Puthamohan VM, Vijayan M. Kathiresan DS, et al. Mol Neurobiol. 2024 Sep 13. doi: 10.1007/s12035-024-04469-x. Online ahead of print. Mol Neurobiol. 2024. PMID: 39269547 Review. - Mutant huntingtin impairs neurodevelopment in human brain organoids through CHCHD2-mediated neurometabolic failure.
Lisowski P, Lickfett S, Rybak-Wolf A, Menacho C, Le S, Pentimalli TM, Notopoulou S, Dykstra W, Oehler D, López-Calcerrada S, Mlody B, Otto M, Wu H, Richter Y, Roth P, Anand R, Kulka LAM, Meierhofer D, Glazar P, Legnini I, Telugu NS, Hahn T, Neuendorf N, Miller DC, Böddrich A, Polzin A, Mayatepek E, Diecke S, Olzscha H, Kirstein J, Ugalde C, Petrakis S, Cambridge S, Rajewsky N, Kühn R, Wanker EE, Priller J, Metzger JJ, Prigione A. Lisowski P, et al. Nat Commun. 2024 Aug 22;15(1):7027. doi: 10.1038/s41467-024-51216-w. Nat Commun. 2024. PMID: 39174523 Free PMC article. - Superoxide dismutase and neurological disorders.
Chidambaram SB, Anand N, Varma SR, Ramamurthy S, Vichitra C, Sharma A, Mahalakshmi AM, Essa MM. Chidambaram SB, et al. IBRO Neurosci Rep. 2024 Jan 23;16:373-394. doi: 10.1016/j.ibneur.2023.11.007. eCollection 2024 Jun. IBRO Neurosci Rep. 2024. PMID: 39007083 Free PMC article. Review. - Mitochondrial targeted antioxidants as potential therapy for huntington's disease.
Upadhayay S, Kumar P. Upadhayay S, et al. Pharmacol Rep. 2024 Aug;76(4):693-713. doi: 10.1007/s43440-024-00619-z. Epub 2024 Jul 9. Pharmacol Rep. 2024. PMID: 38982016 Review. - Defective mitochondria-lysosomal axis enhances the release of extracellular vesicles containing mitochondrial DNA and proteins in Huntington's disease.
Beatriz M, Vilaça R, Anjo SI, Manadas B, Januário C, Rego AC, Lopes C. Beatriz M, et al. J Extracell Biol. 2022 Oct 14;1(10):e65. doi: 10.1002/jex2.65. eCollection 2022 Oct. J Extracell Biol. 2022. PMID: 38939215 Free PMC article.
References
- Folstein S.E. Huntington's Disease. Johns Hopkins University Press; 1990.
- Ross C.A., Tabrizi S.J. Huntington's disease: from molecular pathogenesis to clinical treatment. Lancet Neurol. 2011;10:83–98. doi:10.1016/S1474-4422(10)70245-3. - DOI - PubMed
- Kirkwood S.C., Su J.L., Conneally P., Foroud T. Progression of symptoms in the early and middle stages of Huntington disease. Arch. Neurol. 2001;58:273–278. doi:10.1001/archneur.58.2.273. - DOI - PubMed
- Mahant N., McCusker E.A., Byth K., Graham S. Huntington Study Group. Huntington's disease: clinical correlates of disability and progression. Neurology. 2003;61:1085–1092. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P51 OD011092/OD/NIH HHS/United States
- RR00163/RR/NCRR NIH HHS/United States
- NS31862/NS/NINDS NIH HHS/United States
- AG028072/AG/NIA NIH HHS/United States
- P30-NS061800/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous