Hyperglycemia promotes tissue plasminogen activator-induced hemorrhage by Increasing superoxide production - PubMed (original) (raw)
Hyperglycemia promotes tissue plasminogen activator-induced hemorrhage by Increasing superoxide production
Seok Joon Won et al. Ann Neurol. 2011 Oct.
Abstract
Objective: Risk of intracerebral hemorrhage is the primary factor limiting use of tissue plasminogen activator (tPA) for stroke. Clinical studies have established an association between admission hyperglycemia and the risk of hemorrhage with tPA use, independent of prior diabetes. Here we used an animal model of tPA-induced reperfusion hemorrhage to determine if this clinical association reflects a true causal relationship.
Methods: Rats underwent 90 minutes of focal ischemia, and tPA infusion was begun 10 minutes prior to vessel reperfusion. Glucose was administered during ischemia to generate blood levels ranging from 5.9 ± 1.8mM (normoglycemia) to 21 ± 2.3mM. In some studies, apocynin was administered to block superoxide production by nicotinamide adenine dinucleotide phosphate (NADPH). Brains were harvested 1 hour or 3 days after reperfusion to evaluate the effects of hyperglycemia and apocynin on oxidative stress, blood-brain barrier breakdown, infarct volume, and hemorrhage volume.
Results: Rats that were hyperglycemic during tPA infusion had diffusely increased blood-brain barrier permeability in the postischemic territory, and a 3- to 5-fold increase in intracerebral hemorrhage volumes. The hyperglycemic rats also showed increased superoxide formation in the brain parenchyma and vasculature during reperfusion. The effects of hyperglycemia on superoxide production, blood-brain barrier disruption, infarct size, and hemorrhage were all attenuated by apocynin.
Interpretation: These findings demonstrate a causal relationship between hyperglycemia and hemorrhage in an animal model of tPA stroke treatment, and suggest that this effect of hyperglycemia is mediated through an increase in superoxide production by NADPH oxidase.
Copyright © 2011 American Neurological Association.
Conflict of interest statement
POTENTIAL CONFLICTS OF INTEREST: Nothing to report.
Figures
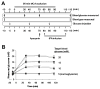
Figure 1. Experimental design
(A) Hyperglycemia was induced by glucose injections at the designated time points. tPA infusion was begun 10 minutes before end of MCA occlusion. Controls received tPA vehicle. (B) Arterial blood glucose concentrations before, during, and after MCA occlusion. Filled symbols, tPA treatment; open symbols, vehicle treatment.
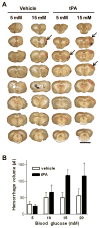
Figure 2. Hyperglycemia increases tPA-induced brain hemorrhage
(A) Each column shows coronal sections of a representative brain from the designated treatment group. Arrows denote some of the hemorrhage areas. Scale bar = 1 cm. (B) Hemorrhage is quantified as the volume of blood per brain. Results from two separate experiments are combined. n = 6–7; P < 0.02 for effects of both glucose and tPA on hemorrhage volume and P < 0.01 for linear trend between glucose and hemorrhage volume in the tPA-treated groups.
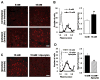
Figure 3. Hyperglycemia-induced superoxide production in re-perfused cortex is blocked by apocynin
(A) Brains were harvested from normoglycemic (5 mM glucose) and hyperglycemic (15 mM glucose) rats 1 hour after reperfusion with tPA. Photomicrographs show superoxide production identified by ethidium fluorescence (red) in representative sections. Scale bar = 40 μm. (B) Histogram shows normalized ethidium fluorescence of cells in cortex of a representative brain from each treatment group, with arrows indicating the median values. Graph shows mean (± s.e.m.) of the median values from the 4 rats in each group; *P < 0.05. (C,D). In a separate experiment, hyperglycemic rats treated were treated with apocynin or vehicle during tPA reperfusion, and brain sections were evaluated as in A,B. n = 5–7; *P < 0.05.
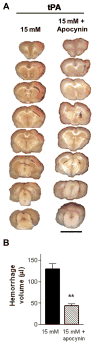
Figure 4. Apocynin reduces hemorrhage in tPA-treated brains
Hyperglycemic rats were pre-treated with apocynin (or vehicle) prior to reperfusion with tPA, and brains were harvested 3 days later. (A) Each column of coronal sections shows a brain from the designated treatment group. Scale bar = 1 cm. (B) Hemorrhage is quantified as volume of blood per brain. n = 6; **P < 0.01.
Figure 5. Effects of hyperglycemia on infarct size and blood-brain barrier
Brains were harvested 3 days after ischemia-reperfusion. (A) Infarct size in tPA-treated rats was increased by hyperglycemia and reduced by apocynin. n = 6–7; * P < 0.05 vs. 5 mM, #P < 0.05 vs. 15 mM. (B) Blood-brain barrier disruption identified by immunostaining for IgG (dark brown) in representative brain sections. (C) Graph shows quantified IgG staining. P < 0.02 for an effect of glucose on IgG staining in both tPA and vehicle-treated brains, with P < 0.01 for linear trend between glucose and IgG staining. (D) Representative images from a separate experiment in which hyperglycemic rats were pre-treated with apocynin or vehicle prior to tPA reperfusion. (E) Graph shows quantified IgG staining. n = 6–7; *P < 0.05. Panels A and C show results combined from 2 separate experiments.
Similar articles
- Considering hyperglycemia and thrombolysis in the Stroke Hyperglycemia Insulin Network Effort (SHINE) trial.
Southerland AM, Johnston KC. Southerland AM, et al. Ann N Y Acad Sci. 2012 Sep;1268(1):72-8. doi: 10.1111/j.1749-6632.2012.06731.x. Ann N Y Acad Sci. 2012. PMID: 22994224 Free PMC article. Review. - Effects of tissue plasminogen activator timing on blood-brain barrier permeability and hemorrhagic transformation in rats with transient ischemic stroke.
Zhang Y, Wang Y, Zuo Z, Wang Z, Roy J, Hou Q, Tong E, Hoffmann A, Sperberg E, Bredno J, Berr SS, Xie M, Lee K, Wintermark M. Zhang Y, et al. J Neurol Sci. 2014 Dec 15;347(1-2):148-54. doi: 10.1016/j.jns.2014.09.036. Epub 2014 Sep 28. J Neurol Sci. 2014. PMID: 25292413 - Effects of admission hyperglycemia on stroke outcome in reperfused tissue plasminogen activator--treated patients.
Alvarez-Sabín J, Molina CA, Montaner J, Arenillas JF, Huertas R, Ribo M, Codina A, Quintana M. Alvarez-Sabín J, et al. Stroke. 2003 May;34(5):1235-41. doi: 10.1161/01.STR.0000068406.30514.31. Epub 2003 Apr 3. Stroke. 2003. PMID: 12677014 - Normobaric hyperoxia reduces the neurovascular complications associated with delayed tissue plasminogen activator treatment in a rat model of focal cerebral ischemia.
Liu W, Hendren J, Qin XJ, Liu KJ. Liu W, et al. Stroke. 2009 Jul;40(7):2526-31. doi: 10.1161/STROKEAHA.108.545483. Epub 2009 May 28. Stroke. 2009. PMID: 19478225 Free PMC article. - Combination approaches to attenuate hemorrhagic transformation after tPA thrombolytic therapy in patients with poststroke hyperglycemia/diabetes.
Fan X, Jiang Y, Yu Z, Yuan J, Sun X, Xiang S, Lo EH, Wang X. Fan X, et al. Adv Pharmacol. 2014;71:391-410. doi: 10.1016/bs.apha.2014.06.007. Epub 2014 Aug 22. Adv Pharmacol. 2014. PMID: 25307224 Review.
Cited by
- Effects of minocycline plus tissue plasminogen activator combination therapy after focal embolic stroke in type 1 diabetic rats.
Fan X, Lo EH, Wang X. Fan X, et al. Stroke. 2013 Mar;44(3):745-52. doi: 10.1161/STROKEAHA.111.000309. Epub 2013 Feb 19. Stroke. 2013. PMID: 23422086 Free PMC article. - Considering hyperglycemia and thrombolysis in the Stroke Hyperglycemia Insulin Network Effort (SHINE) trial.
Southerland AM, Johnston KC. Southerland AM, et al. Ann N Y Acad Sci. 2012 Sep;1268(1):72-8. doi: 10.1111/j.1749-6632.2012.06731.x. Ann N Y Acad Sci. 2012. PMID: 22994224 Free PMC article. Review. - Tissue Plasminogen Activator Promotes TXNIP-NLRP3 Inflammasome Activation after Hyperglycemic Stroke in Mice.
Ismael S, Nasoohi S, Yoo A, Ahmed HA, Ishrat T. Ismael S, et al. Mol Neurobiol. 2020 Jun;57(6):2495-2508. doi: 10.1007/s12035-020-01893-7. Epub 2020 Mar 14. Mol Neurobiol. 2020. PMID: 32172516 Free PMC article. - Hyperglycemia accentuates persistent "functional uncoupling" of cerebral microvascular nitric oxide and superoxide following focal ischemia/reperfusion in rats.
Fabian RH, Kent TA. Fabian RH, et al. Transl Stroke Res. 2012 Dec;3(4):482-90. doi: 10.1007/s12975-012-0210-9. Epub 2012 Sep 5. Transl Stroke Res. 2012. PMID: 24323834
References
- NINDS rt-PA Study Group. Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581–1587. - PubMed
- Demchuk AM, Morgenstern LB, Krieger DW, et al. Serum glucose level and diabetes predict tissue plasminogen activator-related intracerebral hemorrhage in acute ischemic stroke. Stroke. 1999;30:34–39. - PubMed
- Bruno A, Levine SR, Frankel MR, et al. Admission glucose level and clinical outcomes in the NINDS rt-PA Stroke Trial. Neurology. 2002;59:669–674. - PubMed
- Alvarez-Sabin J, Molina CA, Montaner J, et al. Effects of admission hyperglycemia on stroke outcome in reperfused tissue plasminogen activator--treated patients. Stroke. 2003;34:1235–1241. - PubMed
- Kase CS, Furlan AJ, Wechsler LR, et al. Cerebral hemorrhage after intra-arterial thrombolysis for ischemic stroke: the PROACT II trial. Neurology. 2001;57:1603–1610. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical