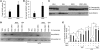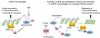Denitrosylation of HDAC2 by targeting Nrf2 restores glucocorticosteroid sensitivity in macrophages from COPD patients - PubMed (original) (raw)
. 2011 Nov;121(11):4289-302.
doi: 10.1172/JCI45144. Epub 2011 Oct 17.
Affiliations
- PMID: 22005302
- PMCID: PMC3204828
- DOI: 10.1172/JCI45144
Denitrosylation of HDAC2 by targeting Nrf2 restores glucocorticosteroid sensitivity in macrophages from COPD patients
Deepti Malhotra et al. J Clin Invest. 2011 Nov.
Retraction in
- Retraction: Denitrosylation of HDAC2 by targeting Nrf2 restores glucocorticosteroid sensitivity in macrophages from COPD patients.
Malhotra D, Thimmulappa RK, Mercado N, Ito K, Kombairaju P, Kumar S, Ma J, Feller-Kopman D, Wise R, Barnes P, Biswal S. Malhotra D, et al. J Clin Invest. 2014 Dec;124(12):5521. doi: 10.1172/JCI79606. Epub 2014 Dec 1. J Clin Invest. 2014. PMID: 25438060 Free PMC article. No abstract available.
Abstract
Chronic obstructive pulmonary disease (COPD), which is caused primarily by cigarette smoking, is a major health problem worldwide. The progressive decline in lung function that occurs in COPD is a result of persistent inflammation of the airways and destruction of the lung parenchyma. Despite the key role of inflammation in the pathogenesis of COPD, treatment with corticosteroids - normally highly effective antiinflammatory drugs - has little therapeutic benefit. This corticosteroid resistance is largely caused by inactivation of histone deacetylase 2 (HDAC2), which is critical for the transrepressive activity of the glucocorticoid receptor (GR) that mediates the antiinflammatory effect of corticosteroids. Here, we show that in alveolar macrophages from patients with COPD, S-nitrosylation of HDAC2 is increased and that this abolishes its GR-transrepression activity and promotes corticosteroid insensitivity. Cys-262 and Cys-274 of HDAC2 were found to be the targets of S-nitrosylation, and exogenous glutathione treatment of macrophages from individuals with COPD restored HDAC2 activity. Treatment with sulforaphane, a small-molecule activator of the transcription factor nuclear factor erythroid 2-related factor 2 (NRF2), was also able to denitrosylate HDAC2, restoring dexamethasone sensitivity in alveolar macrophages from patients with COPD. These effects of sulforaphane were glutathione dependent. We conclude that NRF2 is a novel drug target for reversing corticosteroid resistance in COPD and other corticosteroid-resistant inflammatory diseases.
Figures
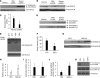
Figure 1. _S_-nitrosylation of HDAC2 protein and concomitant decline in HDAC2 activity in the lungs of patients with COPD.
(A) Levels of _S_-nitrosylated HDAC2 in peripheral lung tissue samples from COPD and non-COPD subjects, as assessed by biotin-switch assay. (B and C) Immunoblot analysis of nuclear HDAC2 protein levels using anti-HDAC2 antibody (B) and enzymatic activity of immunoprecipitated HDAC2 (C) in peripheral lung tissue samples from COPD (n = 6) and non-COPD (n = 3) subjects. (D) Immunoblot analysis of tyrosine nitration (anti-NO-Tyr antibody) and cysteine _S_-nitrosylation (anti-_S_NO antibody) modification of immunoprecipitated HDAC2 from peripheral lung tissues of COPD (n = 6) and non-COPD (n = 3) subjects. (E and F) _S_-nitrosylated HDAC2 levels in alveolar macrophages from patients with COPD, as assessed by a modified biotin-switch assay. (E) Representative blot. (F) Mean band intensity for 6 patients. (G–I) Immunoblot analysis (G) and densitometric quantification (H) of total nuclear HDAC2 protein, and HDAC2 enzymatic activity (I) using immunoprecipitated HDAC2, in COPD alveolar macrophages after treatment with vehicle (VEH) or proteasome inhibitor MG132 (n = 4 per group). (C and I) Enzymatic HDAC2 activity was expressed as “μM of standard.” (J and K) HDAC2 enzymatic activity (J) and HDAC2 protein modification by immunoblot analysis (K) in COPD alveolar macrophages after treatment with GSH-e. Immunoprecipitated HDAC2 from vehicle-treated COPD macrophages was incubated with or without reducing agent DTT as a positive control. *P < 0.01, Student’s t test.
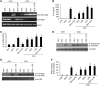
Figure 2. Exposure to CSC induces _S_-nitrosylation of HDAC2 and causes dexamethasone resistance in THP-1 cells overexpressing the Myc-DDK–tagged HDAC2 plasmid.
THP-1 cells were exposed to 200 μg/ml CSC for 24 hours, then to 10 μg/ml LPS for 4 hours. Cells were exposed to 1 μM dexamethasone (DEX) 0.5 hours before LPS exposure. (A) Histone H4 acetylation and HDAC2 binding to IL-8 promoter, as assessed by CHIP assay using anti–pan–H4 acetyl antibody and anti-DDK antibody. (B and C) IL-8 mRNA and protein content, as determined by quantitative RT-PCR and ELISA, respectively. RFC, relative fold change. (D–F) _S_-nitrosylation of HDAC2, determined by biotin-switch assay (D), immunoblotting with anti-S_NO antibody (E), and Saville assay (F). Each assay was conducted in triplicate and also repeated at least 3 times. *P < 0.01 versus vehicle, †_P < 0.01 versus LPS only, Student’s t test.
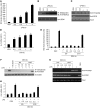
Figure 3. Dexamethasone treatment after LPS exposure fails to suppress NO-dependent _S_-nitrosylation of HDAC2 and IL-8 expression.
THP-1 cells were (A–D) exposed to 10 μg/ml LPS for 4 hours or (E–H) treated with dexamethasone 1, 0.5, and 0.2 hours before and after LPS exposure. (A and E) Levels of NO, assessed by use of DAF-FM diacetate dye. (B and F) _S-_nitrosylation of HDAC2, assessed by biotin-switch assay. (C and G) Histone H4 acetylation and HDAC2 binding to the IL-8 promoter, determined by CHIP assays using anti–pan-H4 acetyl antibody and anti-DDK antibody. (D and H) IL-8 mRNA levels, measured at 0.2, 0.5, 1, and 4 hours (D) or 4 hours (H) after LPS stimulation. Each assay was conducted in triplicate and repeated at least 3 times. *P < 0.01 versus vehicle, Student’s t test.
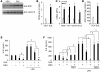
Figure 4. Treatment with exogenous glutathione reverses HDAC2 _S_-nitrosylation of and restores HDAC2 activity and dexamethasone sensitivity in CSC-exposed THP-1 cells.
THP-1 cells were exposed to 200 μg/ml CSC for 24 hours, then with 0.5 mM GSH-e for 2 hours. Cells were then incubated with or without dexamethasone for 1 hour, and then 10 μg/ml LPS for an additional 4 hours. (A) Immunoblot analysis of _S_-nitrosylation and GR binding in coimmunoprecipitated HDAC2, using anti-_S_NO antibody and anti-GR antibody. (B) HDAC2 activity assay. Enzymatic HDAC2 activity was expressed as “μM of standard.” (C) Immunoblot analysis of nitrotyrosine modification and _S_-nitrosylation of HDAC2, detected using anti-nitrotyrosine and anti-S_NO antibodies, respectively. (D) IL-8 expression. Each assay was conducted in triplicate and repeated at least 3 times. *P < 0.01 versus vehicle, #P < 0.01 versus CSC only, †_P < 0.01 versus LPS only, Student’s t test.
Figure 5. Cys-262 and Cys-274 of HDAC2 protein undergo _S_-nitrosylation after exposure to CSC in THP-1 cells.
THP-1 cells overexpressing WT, DEL1, DEL2, and DEL3 constructs were exposed to GSNO. (A) _S_-nitrosylation of immunoprecipitated WT or truncated mutants of HDAC2, as detected by biotin-switch assay. (B and C) THP-1 cells were transfected with WT form or 1 of the site-directed mutants of HDAC2, then exposed to CSC for 24 hours, and _S_-nitrosylation was assessed by biotin-switch assay (B) and enzymatic activity (C). Ascorbate, UV, or HgCl2 was used as a reducing agent to reverse S_-nitrosylation and to demonstrate the specificity of biotin-switch assay. Enzymatic HDAC2 activity was expressed as “μM of standard.” Each assay was conducted in triplicate and repeated at least 3 times. *P < 0.01 versus vehicle WT; †_P < 0.01, CSC versus WT, Student’s t test.
Figure 6. Sulforaphane, by activating Nrf2, suppresses _S_-nitrosylation and restores HDAC2 activity in the alveolar macrophages and lungs of mice exposed to CS.
(A and B) After CS exposure, mice were treated daily with sulforaphane for 3 days (0.5 mg/mouse). Alveolar macrophages and lungs were isolated 24 hours after the last dose of sulforaphane. Alveolar macrophages were then incubated with dexamethasone for 1 hour, followed by LPS exposure for an additional 4 hours. Secretion of IL-6 (A) and MCP-1 (B) was measured in the cell-free culture medium by ELISA. (C and D) Enzymatic HDAC2 activity was expressed as “μM of standard.” Enzymatic activity of total HDAC (C) and HDAC2 (D); GSH levels (E) and S_-nitrosylated HDAC2 levels, as determined by Saville assay (F) and biotin-switch assay (G), were analyzed in lung lysates (n = 6 mice). Immunoblot data are representative of 3 independent experiments. *P < 0.01 versus vehicle, ANOVA followed by Bonferroni post-test. †_P < 0.01 versus respective Nrf2+/+, ‡P < 0.01 as shown by brackets, Student’s t test. Unless otherwise indicated, a total of 6 samples was used.
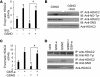
Figure 7. Sulforaphane increases Nrf2-dependent antioxidant defenses and improves corticosteroid responsiveness in alveolar macrophages.
Nuclear extracts of alveolar macrophages were treated with 5 μM sulforaphane (SUL) for 16 hours or with vehicle. (A) Immunoblot analysis of the Nrf2 protein (n = 3 patients) and (B) densitometry analysis of immunoblots normalized to lamin B (n = 9 patients). (C) mRNA analysis of Nrf2-regulated antioxidant genes (n = 9 patients). (D) GSH levels (n = 9 patients). (E) Basal and LPS-induced secretory levels of IL-8 after incubation with or without dexamethasone (DEX) (n = 22 patients). (F) Basal and LPS-induced secretory levels of IL-8 after incubation with or without dexamethasone in the presence or absence of 1 mM BSO for 16 hours. *P < 0.01 versus vehicle, ANOVA followed by Bonferroni post-test. †P < 0.01 versus dexamethasone (with and without LPS); ‡P < 0.01 as shown by brackets, Student’s t test. All assays were repeated 3 times.
Figure 8. Sulforaphane improves corticosteroid sensitivity by increasing HDAC2 activity in a GSH-dependent manner.
(A) Total HDAC activity and (B) HDAC2 enzymatic activity in alveolar macrophages treated with or without 5 μM sulforaphane for 16 hours. Enzymatic HDAC2 activity was expressed as “μM of standard.” (C) HDAC2 S_-nitrosylation, assessed by biotin-switch assay, in COPD alveolar macrophages after sulforaphane treatment in the presence of 1 mM BSO for 16 hours. (D) ChIP analysis of HDAC2 binding and histone acetylation in the IL-8 promoter of alveolar macrophages after sulforaphane cotreatment with 10 mM TSA in ethanol for 2 hours or with 1 mM BSO for 16 hours in the presence or absence of dexamethasone. (E) Basal and LPS-induced histone acetylation in the promoter of the IL-8 gene in alveolar macrophages after coexposure to sulforaphane and 10 mM TSA in ethanol for 2 hours in the presence or absence of dexamethasone. *P < 0.01 versus vehicle, ANOVA followed by Bonferroni post-test. ‡_P < 0.01 as denoted by arrows, Student’s t test. n = 22 patient samples unless otherwise indicated.
Figure 9. Sulforaphane or exogenous GSH increases HDAC2 activity in COPD alveolar macrophages exposed to GSNO.
(A) Enzymatic activity of HDAC2 in GSNO-exposed (0.5 mM, 2 hours) COPD alveolar macrophages after sulforaphane treatment (n = 6 per group). (B) Immunoblot analysis of nitrotyrosine modification and _S_-nitrosylation of HDAC2 in GSNO-exposed alveolar macrophages after treatment with sulforaphane. (C) Enzymatic activity of HDAC2 in GSNO-exposed alveolar macrophages after GSH-e treatment (0.5 mM, 2 hours) (n = 6 per group). (D) Immunoblot analysis of nitrotyrosine modification and S_-nitrosylation of HDAC2 in GSNO-exposed alveolar macrophages after GSH-e treatment. (A and C) Enzymatic HDAC2 activity was expressed as “μM of standard.” *P < 0.01 versus vehicle, ANOVA followed by Bonferroni post-test. ‡_P < 0.01 as shown by brackets, Student’s t test.
Figure 10. Schematic summarizing the study.
_S_-nitrosylation impairs HDAC2-GR binding and HDAC2-DNA binding function. Activation of Nrf2 by sulforaphane increases GSH, which induces denitrosylation of HDAC2 and restores HDAC2 function and thereby GC sensitivity.
Comment in
- Findings of Research Misconduct.
[No authors listed] [No authors listed] Fed Regist. 2019 Nov 14;84(220):61916-61917. Fed Regist. 2019. PMID: 31749507 Free PMC article. No abstract available.
Similar articles
- Targeting Nrf2 signaling improves bacterial clearance by alveolar macrophages in patients with COPD and in a mouse model.
Harvey CJ, Thimmulappa RK, Sethi S, Kong X, Yarmus L, Brown RH, Feller-Kopman D, Wise R, Biswal S. Harvey CJ, et al. Sci Transl Med. 2011 Apr 13;3(78):78ra32. doi: 10.1126/scitranslmed.3002042. Sci Transl Med. 2011. PMID: 21490276 Free PMC article. - Update on molecular mechanisms of corticosteroid resistance in chronic obstructive pulmonary disease.
Jiang Z, Zhu L. Jiang Z, et al. Pulm Pharmacol Ther. 2016 Apr;37:1-8. doi: 10.1016/j.pupt.2016.01.002. Epub 2016 Jan 22. Pulm Pharmacol Ther. 2016. PMID: 26805715 Review. - Histone deacetylase 2-mediated deacetylation of the glucocorticoid receptor enables NF-kappaB suppression.
Ito K, Yamamura S, Essilfie-Quaye S, Cosio B, Ito M, Barnes PJ, Adcock IM. Ito K, et al. J Exp Med. 2006 Jan 23;203(1):7-13. doi: 10.1084/jem.20050466. Epub 2005 Dec 27. J Exp Med. 2006. PMID: 16380507 Free PMC article. - Restoration of HDAC2 and Nrf2 by andrographolide overcomes corticosteroid resistance in chronic obstructive pulmonary disease.
Liao W, Lim AYH, Tan WSD, Abisheganaden J, Wong WSF. Liao W, et al. Br J Pharmacol. 2020 Aug;177(16):3662-3673. doi: 10.1111/bph.15080. Epub 2020 Jun 1. Br J Pharmacol. 2020. PMID: 32335896 Free PMC article. - Role of HDAC2 in the pathophysiology of COPD.
Barnes PJ. Barnes PJ. Annu Rev Physiol. 2009;71:451-64. doi: 10.1146/annurev.physiol.010908.163257. Annu Rev Physiol. 2009. PMID: 18817512 Review.
Cited by
- Prolonged sulforaphane treatment does not enhance tumorigenesis in oncogenic K-ras and xenograft mouse models of lung cancer.
Kombairaju P, Ma J, Thimmulappa RK, Yan SG, Gabrielson E, Singh A, Biswal S. Kombairaju P, et al. J Carcinog. 2012;11:8. doi: 10.4103/1477-3163.98459. Epub 2012 Jul 13. J Carcinog. 2012. PMID: 22919281 Free PMC article. - NRF2-mediated Notch pathway activation enhances hematopoietic reconstitution following myelosuppressive radiation.
Kim JH, Thimmulappa RK, Kumar V, Cui W, Kumar S, Kombairaju P, Zhang H, Margolick J, Matsui W, Macvittie T, Malhotra SV, Biswal S. Kim JH, et al. J Clin Invest. 2014 Feb;124(2):730-41. doi: 10.1172/JCI70812. Epub 2014 Jan 27. J Clin Invest. 2014. PMID: 24463449 Free PMC article. - Molecular mechanisms of repeated social defeat-induced glucocorticoid resistance: Role of microRNA.
Jung SH, Wang Y, Kim T, Tarr A, Reader B, Powell N, Sheridan JF. Jung SH, et al. Brain Behav Immun. 2015 Feb;44:195-206. doi: 10.1016/j.bbi.2014.09.015. Epub 2014 Oct 12. Brain Behav Immun. 2015. PMID: 25317829 Free PMC article. - Nitrosothiols in the immune system: signaling and protection.
Hernansanz-Agustín P, Izquierdo-Álvarez A, García-Ortiz A, Ibiza S, Serrador JM, Martínez-Ruiz A. Hernansanz-Agustín P, et al. Antioxid Redox Signal. 2013 Jan 20;18(3):288-308. doi: 10.1089/ars.2012.4765. Epub 2012 Aug 17. Antioxid Redox Signal. 2013. PMID: 22746191 Free PMC article. Review. - Challenges and Current Efforts in the Development of Biomarkers for Chronic Inflammatory and Remodeling Conditions of the Lungs.
Grunig G, Baghdassarian A, Park SH, Pylawka S, Bleck B, Reibman J, Berman-Rosenzweig E, Durmus N. Grunig G, et al. Biomark Insights. 2016 Feb 16;10(Suppl 4):59-72. doi: 10.4137/BMI.S29514. eCollection 2015. Biomark Insights. 2016. PMID: 26917944 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- U01 HL105569/HL/NHLBI NIH HHS/United States
- P50ES015903/ES/NIEHS NIH HHS/United States
- P50HL107169/HL/NHLBI NIH HHS/United States
- P01 ES018176/ES/NIEHS NIH HHS/United States
- R01 HL081205/HL/NHLBI NIH HHS/United States
- P50 HL084945/HL/NHLBI NIH HHS/United States
- P50 CA058184/CA/NCI NIH HHS/United States
- U01HL105569/HL/NHLBI NIH HHS/United States
- P50HL084945/HL/NHLBI NIH HHS/United States
- P50 HL107169/HL/NHLBI NIH HHS/United States
- P30 ES003819/ES/NIEHS NIH HHS/United States
- P50 ES015903/ES/NIEHS NIH HHS/United States
- P01ES018176/ES/NIEHS NIH HHS/United States
- ES03819/ES/NIEHS NIH HHS/United States
- HL081205/HL/NHLBI NIH HHS/United States