A neutralizing human monoclonal antibody protects african green monkeys from hendra virus challenge - PubMed (original) (raw)
. 2011 Oct 19;3(105):105ra103.
doi: 10.1126/scitranslmed.3002901.
Thomas W Geisbert, Heinz Feldmann, Zhongyu Zhu, Friederike Feldmann, Joan B Geisbert, Lianying Yan, Yan-Ru Feng, Doug Brining, Dana Scott, Yanping Wang, Antony S Dimitrov, Julie Callison, Yee-Peng Chan, Andrew C Hickey, Dimiter S Dimitrov, Christopher C Broder, Barry Rockx
Affiliations
- PMID: 22013123
- PMCID: PMC3313625
- DOI: 10.1126/scitranslmed.3002901
A neutralizing human monoclonal antibody protects african green monkeys from hendra virus challenge
Katharine N Bossart et al. Sci Transl Med. 2011.
Abstract
Hendra virus (HeV) is a recently emerged zoonotic paramyxovirus that can cause a severe and often fatal disease in horses and humans. HeV is categorized as a biosafety level 4 agent, which has made the development of animal models and testing of potential therapeutics and vaccines challenging. Infection of African green monkeys (AGMs) with HeV was recently demonstrated, and disease mirrored fatal HeV infection in humans, manifesting as a multisystemic vasculitis with widespread virus replication in vascular tissues and severe pathologic manifestations in the lung, spleen, and brain. Here, we demonstrate that m102.4, a potent HeV-neutralizing human monoclonal antibody (hmAb), can protect AGMs from disease after infection with HeV. Fourteen AGMs were challenged intratracheally with a lethal dose of HeV, and 12 subjects were infused twice with a 100-mg dose of m102.4 beginning at either 10, 24, or 72 hours after infection and again about 48 hours later. The presence of viral RNA, infectious virus, and HeV-specific immune responses demonstrated that all subjects were infected after challenge. All 12 AGMs that received m102.4 survived infection, whereas the untreated control subjects succumbed to disease on day 8 after infection. Animals in the 72-hour treatment group exhibited neurological signs of disease, but all animals started to recover by day 16 after infection. These results represent successful post-exposure in vivo efficacy by an investigational drug against HeV and highlight the potential impact a hmAb can have on human disease.
Conflict of interest statement
Competing interests: C.C.B. and D.S.D. are United States federal employees, and D.S.D., Z.Z. and C.C.B. are coinventors on United States patent 7,988,971, Human monoclonal antibodies against Hendra and Nipah viruses; assignees are The United States of America as represented by the Department of Health and Human Services (Washington, DC), Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. (Bethesda, MD). All other authors declare no competing interests. The opinions or assertions contained herein are the private ones of the author(s) and are not to be construed as official or reflecting the views of the Department of Defense, the Uniformed Services University of Health Sciences, and the National Institutes of Allergy and Infectious Diseases, National Institutes of Health.
Figures
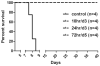
Figure 1. Survival curve of HeV-infected monkeys
Data from control subjects and m102.4-treated subjects were used to generate the Kaplan-Meier survival curve. Control included data from two additional historical control subjects (28). Subjects received m102.4 infusions at 10 hours and 3 days p.i. (10hr/d3), 24 hrs and 3 days p.i. (24hr/d3) or 72 hrs and 5 days p.i. (72hr/d5). Each group contained 4 subjects (n=4). Average time to end stage disease was 8 days in control subjects whereas all m102.4 treated subjects survived until euthanasia at the end of the study.
Figure 2. HeV antigen and viral RNA in untreated control AGM tissues and viral RNA in blood from m102.4 efficacy trial
(A) Localization of HeV antigen by immunohistochemical stain in the lung and brainstem of AGM 13. Sections were stained with a NiV nucleoprotein (N) specific rabbit polyclonal antibody and images were obtained at an original magnification of at 40X; however one panel was photographed at 200X as indicated. (B) Detection of HeV RNA in tissues collected from AGM 13 and AGM 17. (C) Detection of HeV RNA in blood samples. RNA samples were assayed in triplicate using TaqMan PCR. Blood and tissue Ct values were analyzed against Ct values generated from a standard curve of HeV RNA, as described in the methods, and a relative HeV N expression value was calculated for each blood replicate. Results are shown as average relative HeV N gene expression levels. The data are mean +/− SD. In panel C data from individual subjects are shown and indicated as none or different hatched patterns, and bar coloration (inset legend) indicates the different treatment groups. Virus isolation was attempted on all tissue and blood samples, and positive samples are indicated by (+). The blood sample (*) from one control subject was a terminal sample taken on day 8; lymph node.
Figure 3. Detection of F-specific antibodies in m102.4 treated AGMs
Median fluorescence intensities (M.F.I.) are shown on the Y-axis and represent binding to soluble F. Error bars represent the standard deviation of fluorescence intensity across 100 beads for each sample. Three of 4 subjects in the 10hr/d3 group were sampled on days 24 and 30 instead of days 27 and 35; the remaining animal ( ) in this group was sampled on day 27 and 35. No sample (*) was collected for one animal (
) in this group was sampled on day 27 and 35. No sample (*) was collected for one animal ( ) on day 24.
) on day 24.
Similar articles
- Therapeutic treatment of Nipah virus infection in nonhuman primates with a neutralizing human monoclonal antibody.
Geisbert TW, Mire CE, Geisbert JB, Chan YP, Agans KN, Feldmann F, Fenton KA, Zhu Z, Dimitrov DS, Scott DP, Bossart KN, Feldmann H, Broder CC. Geisbert TW, et al. Sci Transl Med. 2014 Jun 25;6(242):242ra82. doi: 10.1126/scitranslmed.3008929. Sci Transl Med. 2014. PMID: 24964990 Free PMC article. - A recombinant Hendra virus G glycoprotein subunit vaccine protects nonhuman primates against Hendra virus challenge.
Mire CE, Geisbert JB, Agans KN, Feng YR, Fenton KA, Bossart KN, Yan L, Chan YP, Broder CC, Geisbert TW. Mire CE, et al. J Virol. 2014 May;88(9):4624-31. doi: 10.1128/JVI.00005-14. Epub 2014 Feb 12. J Virol. 2014. PMID: 24522928 Free PMC article. - A novel model of lethal Hendra virus infection in African green monkeys and the effectiveness of ribavirin treatment.
Rockx B, Bossart KN, Feldmann F, Geisbert JB, Hickey AC, Brining D, Callison J, Safronetz D, Marzi A, Kercher L, Long D, Broder CC, Feldmann H, Geisbert TW. Rockx B, et al. J Virol. 2010 Oct;84(19):9831-9. doi: 10.1128/JVI.01163-10. Epub 2010 Jul 21. J Virol. 2010. PMID: 20660198 Free PMC article. - Animal challenge models of henipavirus infection and pathogenesis.
Geisbert TW, Feldmann H, Broder CC. Geisbert TW, et al. Curr Top Microbiol Immunol. 2012;359:153-77. doi: 10.1007/82_2012_208. Curr Top Microbiol Immunol. 2012. PMID: 22476556 Free PMC article. Review. - Hendra Virus Infection in Horses: A Review on Emerging Mystery Paramyxovirus.
Khusro A, Aarti C, Pliego AB, Cipriano-Salazar M. Khusro A, et al. J Equine Vet Sci. 2020 Aug;91:103149. doi: 10.1016/j.jevs.2020.103149. Epub 2020 May 30. J Equine Vet Sci. 2020. PMID: 32684248 Review.
Cited by
- Towards global health security: response to the May 2018 Nipah virus outbreak linked to Pteropus bats in Kerala, India.
Sadanadan R, Arunkumar G, Laserson KF, Heretik KH, Singh S, Mourya DT, Gangakhedkar RR, Gupta N, Sharma R, Dhuria M, Jain SK, Nichol S, Gupta P, Bhargava B. Sadanadan R, et al. BMJ Glob Health. 2018 Nov 9;3(6):e001086. doi: 10.1136/bmjgh-2018-001086. eCollection 2018. BMJ Glob Health. 2018. PMID: 30483413 Free PMC article. No abstract available. - Combinatorial F-G Immunogens as Nipah and Respiratory Syncytial Virus Vaccine Candidates.
Isaacs A, Cheung STM, Thakur N, Jaberolansar N, Young A, Modhiran N, Bailey D, Graham SP, Young PR, Chappell KJ, Watterson D. Isaacs A, et al. Viruses. 2021 Sep 28;13(10):1942. doi: 10.3390/v13101942. Viruses. 2021. PMID: 34696372 Free PMC article. - Nipah Virus: Past Outbreaks and Future Containment.
Soman Pillai V, Krishna G, Valiya Veettil M. Soman Pillai V, et al. Viruses. 2020 Apr 20;12(4):465. doi: 10.3390/v12040465. Viruses. 2020. PMID: 32325930 Free PMC article. Review. - A treatment for and vaccine against the deadly Hendra and Nipah viruses.
Broder CC, Xu K, Nikolov DB, Zhu Z, Dimitrov DS, Middleton D, Pallister J, Geisbert TW, Bossart KN, Wang LF. Broder CC, et al. Antiviral Res. 2013 Oct;100(1):8-13. doi: 10.1016/j.antiviral.2013.06.012. Epub 2013 Jul 6. Antiviral Res. 2013. PMID: 23838047 Free PMC article. Review. - Prefusion stabilization of the Hendra and Langya virus F proteins.
Byrne PO, Blade EG, Fisher BE, Ambrozak DR, Ramamohan AR, Graham BS, Loomis RJ, McLellan JS. Byrne PO, et al. J Virol. 2024 Feb 20;98(2):e0137223. doi: 10.1128/jvi.01372-23. Epub 2024 Jan 12. J Virol. 2024. PMID: 38214525 Free PMC article.
References
- Mayo MA. A summary of taxonomic changes recently approved by ICTV. Arch Virol. 2002;147:1655. - PubMed
- Field H, Young P, Yob JM, Mills J, Hall L, Mackenzie J. The natural history of Hendra and Nipah viruses. Microbes Infect. 2001;3:307. - PubMed
- Bossart KN, Tachedjian M, McEachern JA, Crameri G, Zhu Z, Dimitrov DS, Broder CC, Wang LF. Functional studies of host-specific ephrin-B ligands as Henipavirus receptors. Virology. 2008;372:357. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 AI077995-04/AI/NIAID NIH HHS/United States
- AI054715/AI/NIAID NIH HHS/United States
- U54 AI057159/AI/NIAID NIH HHS/United States
- U01 AI082121-01/AI/NIAID NIH HHS/United States
- R01 AI054715/AI/NIAID NIH HHS/United States
- AI082121/AI/NIAID NIH HHS/United States
- R01 AI054715-05/AI/NIAID NIH HHS/United States
- U54 AI057159-06/AI/NIAID NIH HHS/United States
- U01 AI082121/AI/NIAID NIH HHS/United States
- AI057159/AI/NIAID NIH HHS/United States
- N01CO12400/CA/NCI NIH HHS/United States
- AI077995/AI/NIAID NIH HHS/United States
- N01-CO-12400/CO/NCI NIH HHS/United States
- U01 AI077995/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources