Inhibition of miR-15 protects against cardiac ischemic injury - PubMed (original) (raw)
. 2012 Jan 6;110(1):71-81.
doi: 10.1161/CIRCRESAHA.111.244442. Epub 2011 Nov 3.
Rusty L Montgomery, Anita G Seto, Brent A Dickinson, Hillary M Semus, Joshua M Lynch, Christina M Dalby, Kathryn Robinson, Christianna Stack, Paul A Latimer, Joshua M Hare, Eric N Olson, Eva van Rooij
Affiliations
- PMID: 22052914
- PMCID: PMC3354618
- DOI: 10.1161/CIRCRESAHA.111.244442
Inhibition of miR-15 protects against cardiac ischemic injury
Thomas G Hullinger et al. Circ Res. 2012.
Abstract
Rationale: Myocardial infarction (MI) is a leading cause of death worldwide. Because endogenous cardiac repair mechanisms are not sufficient for meaningful tissue regeneration, MI results in loss of cardiac tissue and detrimental remodeling events. MicroRNAs (miRNAs) are small, noncoding RNAs that regulate gene expression in a sequence dependent manner. Our previous data indicate that miRNAs are dysregulated in response to ischemic injury of the heart and actively contribute to cardiac remodeling after MI.
Objective: This study was designed to determine whether miRNAs are dysregulated on ischemic damage in porcine cardiac tissues and whether locked nucleic acid (LNA)-modified anti-miR chemistries can target cardiac expressed miRNAs to therapeutically inhibit miR-15 on ischemic injury.
Methods and results: Our data indicate that the miR-15 family, which includes 6 closely related miRNAs, is regulated in the infarcted region of the heart in response to ischemia-reperfusion injury in mice and pigs. LNA-modified chemistries can effectively silence miR-15 family members in vitro and render cardiomyocytes resistant to hypoxia-induced cardiomyocyte cell death. Correspondingly, systemic delivery of miR-15 anti-miRs dose-dependently represses miR-15 in cardiac tissue of both mice and pigs, whereas therapeutic targeting of miR-15 in mice reduces infarct size and cardiac remodeling and enhances cardiac function in response to MI.
Conclusions: Oligonucleotide-based therapies using LNA-modified chemistries for modulating cardiac miRNAs in the setting of heart disease are efficacious and validate miR-15 as a potential therapeutic target for the manipulation of cardiac remodeling and function in the setting of ischemic injury.
Figures
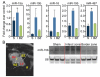
Figure 1. miR-15 family is upregulated in the infarcted region of porcine cardiac tissue in response to ischemic injury
A, Real-time PCR analysis indicates that the miR-15 family is upregulated in the infarct zone in porcine cardiac tissue 24 hours after ischemia-reperfusion. miR-15a, miR-195, and miR-497, *P<0.05 versus border zone; miR-15b, _P_=0.13; miR-195, _P_=0.09 (ANOVA); n=3 per group. S indicates sham; LV, left ventricle; RV, right ventricle; I, infarct; BZ, border zone. B, MRI cross-sectional image of porcine heart demonstrating I, BZ, normally perfused remote region (R), LV, and RV. Northern blot analysis of porcine cardiac tissue from these areas indicates an upregulation of miR-15b specifically in the infarcted region 24 hours after ischemia-reperfusion.
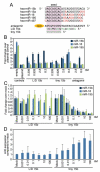
Figure 2. Anti-miR-mediated silencing of miR-15 family members in vitro
A, miR-15 family sequences and anti-miR designs. Antagomir 15b is directed against miR-15b, and contains the full length complementary reverse sequence of the mature miRNA in which all nucleosides are 2′-Ome modified with two 5′ terminal and four 3′ terminal bases containing a phosphorothioate internucleoside bond and a 3′ cholesterol (chol) attached through a hydroxyprolinol linker. The 16-mer anti-miR is an unconjugated LNA/DNA anti-miR complementary to the 5′ end of miR-15b (L/D 15b), whereas the 8-mer LNA-anti-miR is complementary to the seed region of the miR-15 family (tiny 15b). The LNA-modified chemistries are fully phosphorothioated (green indicates LNA). B, Luciferase assays in Hela cells using reporters harboring a perfect binding site for the different miR-15 members (miR-15b, −16, and −195) indicated a dose-responsive derepression of the miR-15b luciferase reporter for all 3 oligonucleotide chemistries tested, with the L/D 15b being most efficacious and the antagomir-15b showing the least activity. As expected based on sequence composition, tiny 15b also represses miR-16 and -195 activity, whereas the L/D 15b preferentially inhibits miR-15b. C, Real-time PCR analysis in cardiomyocytes shows that L/D 15b potently inhibits miR-15b, whereas tiny 15b inhibits multiple family members (empty indicates plasmid without target site; plasmid, plasmid with target site). D, Real-time PCR analysis for Arl2, a defined miR-15 target, shows a dose-dependent derepression in response to increasing doses of tiny 15b, whereas this response is absent in the L/D treated cells. B through D show average results for 3 independent experiments.
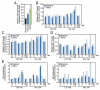
Figure 3. miR-15 inhibition increases cardiomyocyte survival under hypoxic conditions
A, miR-15b levels increase in cardiomyocytes in response to hypoxia, which is further enhanced by subsequent reoxygenation (*P<0.05 versus normoxia by ANOVA). B, Cardiomyocyte survival, as determined by relative ATP levels, increases dose-dependently in response to tiny 15b compared with control, whereas this effect is absent for L/D 15b (Ctrl indicates control oligonucleotide, *P<0.05 versus respective control by ANOVA). C, Measuring numbers of cells through total cytoplasmic lactate dehydrogenase indicates that tiny 15b dose-dependently increases cell viability during hypoxia and hypoxia/reoxygenation. (Ctrl indicates control oligonucleotide,*P<0.05 versus respective control by ANOVA). D, Using the MTT assay as a measure of cell viability shows that tiny 15b dose-dependently increases cell viability compared with control treatment, especially under conditions of hypoxia/reoxygenation (Ctrl indicates control oligonucleotide, *P<0.05 versus respective control by ANOVA). E, Real-time PCR analysis indicates that both L/D 15b and tiny 15b dose-dependently increase the miR-15 target Arl2 compared with control; however, this effect is most pronounced after tiny 15b treatment under hypoxic conditions. Real-time PCR analysis for Bcl2 shows a moderate increase after L/D 15b treatment during reoxygenation; however, this effect is significantly more pronounced in response to tiny 15b treatment (Ctrl indicates control oligonucleotide, *P<0.05 versus respective control by ANOVA). Figure represents average data from 3 independent experiments.
Figure 4. Cardiac silencing of miR-15 in mice using an LNA-modified anti-miR
A, Real-time PCR analysis for miR-15b 1 week after intravenous administration of increasing doses of L/D 15b or equalmolar amounts of tiny 15b indicates potent silencing of cardiac miR-15b. B, Real-time PCR analysis 1 week after systemic administration of either L/D 15b or equal molar amounts of tiny 15b shows effective knockdown for multiple miR-15 family members in response to angiotensin II treatment. As expected based on sequence composition, tiny 15b also represses miR-16 and −195 activity, whereas L/D 15b preferentially inhibits miR-15b. C, Northern blotting confirms a dose-dependent cardiac silencing of miR-16. The detectable upshift of miR-16 in the presence of tiny 15b reflects the formation of a stable heteroduplex between miR-16 and the LNA-anti-miR. Inhibition of miR-16 in response to tiny 15b indicates a more pronounced inhibition by Northern analysis, because the real-time PCR procedure disrupts the binding between the anti-miR and tiny 15b, presenting an underrepresentation of anti-miR-miRNA interaction. D, Plasma detection of L/D 15b shows a rapid (6 -12 hours) elimination phase, after which small amounts of anti-miR remain detectable in the plasma for at least 7 days in a dose-dependent manner. E, L/D 15b detection in cardiac tissues indicates a dose-dependent presence of anti-miR, which remains fairly stable between days 1 and 7. F, The detection of L/D 15b in cardiac tissue 1 week after administration is dose-dependent. G, Tissue detection in heart, liver, and kidney shows that considerable amounts of L/D 15b target the liver and kidney. Data represent the average of n=4 per group.
Figure 5. miR-15 inhibition in porcine cardiac tissue
A, Real-time PCR analysis 4 days after intravenous administration of increasing doses of L/D 15b indicates potent silencing of cardiac miR-15b. B, Northern blotting confirms a dose-dependent cardiac silencing of miR-15b and miR-16 in porcine cardiac tissue 4 days after dosing. L/D 15b preferentially target miR-15b. C, Real-time PCR analysis both 1 and 4 days after intravenous administration of increasing doses of tiny 15b indicates potent silencing of cardiac miR-15b. D, Northern blotting confirms a dose-dependent cardiac silencing of miR-15b and miR-16 in porcine cardiac tissue in response to tiny 15b treatment. The observed upshift reflects the formation of a stable heteroduplex between the mature miRNAs and the LNA-anti-miR. E, Cardiac detection of L/D 15b and tiny 15b shows a relatively equal distribution of the anti-miR across the heart that is dose-responsive for both anti-miRs, with no differences in detectable amount of anti-miR between days 1 and 4 after tiny 15b administration. When 2 doses are indicated, the first dose is tiny 15b and second dose is L/D 15b. LV indicates left ventricle; RV, right ventricle; and Sept, interventricular septum. Data represent the average of n=3 per group.
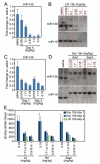
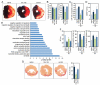
Figure 6. miR-15 inhibition reduces infarct size and improves function in response to ischemia
A, Representative images after TTC staining indicate that although the area at risk (AAR, red and white) is comparable between the different treatment groups, the infarcted area (IA, white) is smaller in the tiny 15b-treated animals (control indicates control oligonucleotide). B, Quantification of cross sections of the infarcted hearts indicate that the AAR is ≈50% of the LV for all 3 treatment groups, whereas administration of 0.5 mg/kg of tiny 15b during reperfusion results in a significant reduction in infarct size compared with either saline or control oligo (*P<0.05 versus saline and control by ANOVA; control indicates control oligonucleotide). C, Real-time PCR analysis on tissue of the ischemic region 24 hours after reperfusion indicates inhibition of miR-15b in response to tiny 15b treatment (*P<0.05 versus saline and control oligonucleotide treated by ANOVA). D, Left ventricular end-diastolic pressure recordings 24 hours after reperfusion reveals an increase with saline treatment and a reduction with tiny 15b treatment (control indicates control oligonucleotide, *P<0.05 versus sham Kruskal-Wallis test). E, Ontology analysis of transcripts upregulated ≥1.5-fold in the ischemic region of hearts 24 hours after reperfusion treated with tiny 15b treatment compared with saline, based on microarray profiling. Negative regulators of apoptosis and cell death are significantly overrepresented. F, Echocardiography shows a reduction in ejection fraction (EF) and increases in LV volumes 2 weeks after infarct, all of which are significantly improved in response to tiny 15b treatment (*P<0.05 versus saline and control by ANOVA for EF and LVESV, versus saline only LVEDV; sham indicates no ischemia/reperfusion; control, control oligo). G, Representative images of Picrosirius red-stained cross sections demonstrate a reduction in collagen content of the left ventricle 2 weeks after reperfusion with tiny 15b treatment. Quantification of fibrosis as a percentage of total left ventricular area reveals a statistically significant reduction in the tiny 15b-treated group (*P<0.05 versus saline-treated by ANOVA). LV indicates left ventricle.
Similar articles
- Inhibition of microRNA-92a protects against ischemia/reperfusion injury in a large-animal model.
Hinkel R, Penzkofer D, Zühlke S, Fischer A, Husada W, Xu QF, Baloch E, van Rooij E, Zeiher AM, Kupatt C, Dimmeler S. Hinkel R, et al. Circulation. 2013 Sep 3;128(10):1066-75. doi: 10.1161/CIRCULATIONAHA.113.001904. Epub 2013 Jul 29. Circulation. 2013. PMID: 23897866 - Inhibition of miR-181b-5p protects cardiomyocytes against ischemia/reperfusion injury by targeting AKT3 and PI3KR3.
Yuan L, Fan L, Li Q, Cui W, Wang X, Zhang Z. Yuan L, et al. J Cell Biochem. 2019 Dec;120(12):19647-19659. doi: 10.1002/jcb.29271. Epub 2019 Jul 11. J Cell Biochem. 2019. PMID: 31297863 - Inhibition of miR-217 Protects Against Myocardial Ischemia-Reperfusion Injury Through Inactivating NF-κB and MAPK Pathways.
Li Y, Fei L, Wang J, Niu Q. Li Y, et al. Cardiovasc Eng Technol. 2020 Apr;11(2):219-227. doi: 10.1007/s13239-019-00452-z. Epub 2020 Jan 8. Cardiovasc Eng Technol. 2020. PMID: 31916040 - Role of microRNAs in the reperfused myocardium towards post-infarct remodelling.
Zhu H, Fan GC. Zhu H, et al. Cardiovasc Res. 2012 May 1;94(2):284-92. doi: 10.1093/cvr/cvr291. Epub 2011 Oct 28. Cardiovasc Res. 2012. PMID: 22038740 Free PMC article. Review. - MicroRNA-133a and Myocardial Infarction.
Xiao Y, Zhao J, Tuazon JP, Borlongan CV, Yu G. Xiao Y, et al. Cell Transplant. 2019 Jul;28(7):831-838. doi: 10.1177/0963689719843806. Epub 2019 Apr 14. Cell Transplant. 2019. PMID: 30983393 Free PMC article. Review.
Cited by
- Unveiling exosomes: Cutting-edge isolation techniques and their therapeutic potential.
Sani F, Shafiei F, Dehghani F, Mohammadi Y, Khorraminejad-Shirazi M, Anvari-Yazdi AF, Moayedfard Z, Azarpira N, Sani M. Sani F, et al. J Cell Mol Med. 2024 Oct;28(20):e70139. doi: 10.1111/jcmm.70139. J Cell Mol Med. 2024. PMID: 39431552 Free PMC article. Review. - Noncoding RNA in age-related cardiovascular diseases.
Greco S, Gorospe M, Martelli F. Greco S, et al. J Mol Cell Cardiol. 2015 Jun;83:142-55. doi: 10.1016/j.yjmcc.2015.01.011. Epub 2015 Jan 29. J Mol Cell Cardiol. 2015. PMID: 25640162 Free PMC article. Review. - Chronic miR-29 antagonism promotes favorable plaque remodeling in atherosclerotic mice.
Ulrich V, Rotllan N, Araldi E, Luciano A, Skroblin P, Abonnenc M, Perrotta P, Yin X, Bauer A, Leslie KL, Zhang P, Aryal B, Montgomery RL, Thum T, Martin K, Suarez Y, Mayr M, Fernandez-Hernando C, Sessa WC. Ulrich V, et al. EMBO Mol Med. 2016 Jun 1;8(6):643-53. doi: 10.15252/emmm.201506031. Print 2016 Jun. EMBO Mol Med. 2016. PMID: 27137489 Free PMC article. - Strategies to Modulate MicroRNA Functions for the Treatment of Cancer or Organ Injury.
Lee TJ, Yuan X, Kerr K, Yoo JY, Kim DH, Kaur B, Eltzschig HK. Lee TJ, et al. Pharmacol Rev. 2020 Jul;72(3):639-667. doi: 10.1124/pr.119.019026. Pharmacol Rev. 2020. PMID: 32554488 Free PMC article. Review. - Regulation of miRNAs by Natural Antioxidants in Cardiovascular Diseases: Focus on SIRT1 and eNOS.
Lee Y, Im E. Lee Y, et al. Antioxidants (Basel). 2021 Mar 3;10(3):377. doi: 10.3390/antiox10030377. Antioxidants (Basel). 2021. PMID: 33802566 Free PMC article. Review.
References
- Cannon RO., III Mechanisms, management and future directions for reperfusion injury after acute myocardial infarction. Nat Clin Pract Cardiovasc Med. 2005;2:88–94. - PubMed
- Wollert KC, Drexler H. Cell therapy for the treatment of coronary heart disease: a critical appraisal. Nat Rev Cardiol. 2010;7:204–215. - PubMed
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL077439/HL/NHLBI NIH HHS/United States
- R01 AR040339/AR/NIAMS NIH HHS/United States
- R01 HL111665/HL/NHLBI NIH HHS/United States
- P50 HL061033/HL/NHLBI NIH HHS/United States
- S10 RR019137/RR/NCRR NIH HHS/United States
- R01 HL093039/HL/NHLBI NIH HHS/United States
- R37 HL053351/HL/NHLBI NIH HHS/United States
- R01 HL083371/HL/NHLBI NIH HHS/United States
- R01 HL061544/HL/NHLBI NIH HHS/United States
- R01 HL053351/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases