Antifibrotic effects of curcumin are associated with overexpression of cathepsins K and L in bleomycin treated mice and human fibroblasts - PubMed (original) (raw)
Antifibrotic effects of curcumin are associated with overexpression of cathepsins K and L in bleomycin treated mice and human fibroblasts
Dongwei Zhang et al. Respir Res. 2011.
Abstract
Background: Lung fibrosis is characterized by fibroblast proliferation and the deposition of collagens. Curcumin, a polyphenol antioxidant from the spice tumeric, has been shown to effectively counteract fibroblast proliferation and reducing inflammation and fibrotic progression in animal models of bleomycin-induced lung injury. However, there is little mechanistic insight in the biological activity of curcumin. Here, we study the effects of curcumin on the expression and activity of cathepsins which have been implicated in the development of fibrotic lung diseases.
Methods: We investigated the effects of curcumin administration to bleomycin stimulated C57BL/6 mice and human fetal lung fibroblasts (HFL-1) on the expression of cathepsins K and L which have been implicated in matrix degradation, TGF-β1 modulation, and apoptosis. Lung tissues were evaluated for their contents of cathepsins K and L, collagen, and TGF-β1. HFL-1 cells were used to investigate the effects of curcumin and cathepsin inhibition on cell proliferation, migration, apoptosis, and the expression of cathepsins K and L and TGF-β1.
Results: Collagen deposition in lungs was decreased by 17-28% after curcumin treatment which was accompanied by increased expression levels of cathepsins L (25%-39%) and K (41%-76%) and a 30% decrease in TGF-β1 expression. Moreover, Tunel staining of lung tissue revealed a 33-41% increase in apoptotic cells after curcumin treatment. These in vivo data correlated well with data obtained from the human fibroblast line, HFL-1. Here, cathepsin K and L expression increased 190% and 240%, respectively, in the presence of curcumin and the expression of TGF-β1 decreased by 34%. Furthermore, curcumin significantly decreased cell proliferation and migration and increased the expression of surrogate markers of apoptosis. In contrast, these curcumin effects were partly reversed by a potent cathepsin inhibitor.
Conclusion: This study demonstrates that curcumin increases the expression of cathepsins K and L in lung which an effect on lung fibroblast cell behavior such as proliferation, migration and apoptosis rates and on the expression of TGF-β1 in mouse lung and HFL-1 cells. These results suggest that cathepsin-inducing drugs such as curcumin may be beneficial in the treatment of lung fibrosis.
Figures
Figure 1
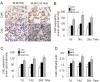
Effect of curcumin on the expression of cathepsins K and L in mouse lungs of bleomycin induced fibrosis. Representative images (A) of immunohistochemical staining and quantification (B and C) of the expression of CatK and L in curcumin treated bleomycin induced mice show that expressions of CatK and L were increased at 7d, 14 d and 28 d. Real time PCR results (D) also show RNA levels of CatK were increased after curcumin and bleomycin treatment at the indicated time points. For each time point 9-10 mice were evaluated. All data represent means ± SE (* p < 0.05, all compared to bleomycin treated group only; Con: control, BLM: bleomycin, Cur: curcumin).
Figure 2
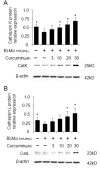
Overexpression of cathepsins K and L in curcumin treated bleomycin stimulated human lung fibroblasts (HFL-1 cells). Representative images (A and B) of immunoblots and measurements show that expressions of CatK and L were dose-dependently increased by curcumin. All data represent means of three independent experiments ± SD and represent percentage cathepsin expression compared to β-actin as control (* p < 0.05, all compared to bleomycin treated group only).
Figure 3
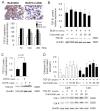
Effects of curcumin on collagen deposition in fibrotic lungs of mice (bleomycin-induced) and HFL-1 cell proliferation and migration. Representative images of Masson trichrome staining (A) and quantitative image analysis (B) of collagen content in the lungs of 9-10 mice/group display a reduction of collagen by curcumin. MTT assay (C) shows curcumin dose-dependent decrease of proliferation of HFL-1 cells in the presence of bleomycin (0.1 mU/ml) at 24, 48 and 72 h. Cell migration rates (D) of HFL-1 cells were also decreased by curcumin. All the cell-related data represent means of three independent experiments ± SD. The Masson trichrome staining evaluation is based on the image analysis of two tissue section per mouse and data represent means ± SE (* p < 0.05, all compared to bleomycin treated group only).
Figure 4
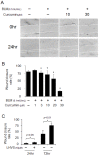
Effects of curcumin and a cathepsin inhibitor on wound healing in HFL-1 cell scratch model. Representative images of wound healing assay (A) and its quantification (B) demonstrate the dose-dependent reduction of wound closure rates by curcumin. In contrast, LHVS, a pan-cathepsin inhibitor significantly increased wound closure rates (C). All data represent means of three independent experiments ± SD (* p < 0.05, all compared to bleomycin treated group only).
Figure 5
Effects of curcumin on expressions of TGF-β1, and the effect of TGF-β1 on cathepsins K and L expression. Representative immunohistochemical images and image analysis (A) of TGF-β1 expression in bleomycin treated mice (9- 10 mice per group). Curcumin significantly decreased TGF-β1 expressions at 14d and 28d. All data besides the TGF-β1 analysis (means ± SE) represent means of three independent experiments ± SD (* p < 0.05, all compared to bleomycin treated group only). (B) Representative immunoblot images and their quantitative analysis display the dose-dependent reduction of TGF-β1 protein content by curcumin in bleomycin treated HFL-1 cells. In contrast, LHVS increased TGF-β1 protein content two-fold (C). Panel D shows a 7 and 5-fold increase in the expressions of CatK and L in TGF-β1 treated HFL-1 cells when exposed to curcumin (20 μM) whereas TGF-β1 alone significantly decreased cathepsin expression. All data represent means of three independent experiments ± SD (* p < 0.05, all compared to bleomycin or TGF-β1 treated group only).
Figure 6
Effects of curcumin and a cathepsin inhibitor on the expression of apoptosis markers in bleomycin stimulated HFL-1 cells. Quantitative analysis of Tunel-positive staining in lungs of curcumin treated bleomycin-induced lung injury (A). Representative immunoblot images and their quantitative analysis (B and C) show that curcumin increases caspase-3, Bax/Bcl-2 expression in a dose-dependent manner. LHVS, a pan-cathepsin inhibitor is able to reverse the proapoptotic effect of curcumin as seen by the reduction of caspase-3 expression and the Bax/Bcl-2 ratio in HFL-1 cells (D). All data represent means of three independent experiments ± SD. (* p < 0.05, all compared to bleomycin treated group only).
Similar articles
- Overexpression of cathepsin K in mice decreases collagen deposition and lung resistance in response to bleomycin-induced pulmonary fibrosis.
Srivastava M, Steinwede K, Kiviranta R, Morko J, Hoymann HG, Länger F, Buhling F, Welte T, Maus UA. Srivastava M, et al. Respir Res. 2008 Jul 18;9(1):54. doi: 10.1186/1465-9921-9-54. Respir Res. 2008. PMID: 18638383 Free PMC article. - Antifibrotic effects of cyclosporine A on TGF-β1-treated lung fibroblasts and lungs from bleomycin-treated mice: role of hypoxia-inducible factor-1α.
Yamazaki R, Kasuya Y, Fujita T, Umezawa H, Yanagihara M, Nakamura H, Yoshino I, Tatsumi K, Murayama T. Yamazaki R, et al. FASEB J. 2017 Aug;31(8):3359-3371. doi: 10.1096/fj.201601357R. Epub 2017 Apr 26. FASEB J. 2017. PMID: 28446589 - Inhibition of Wnt/β-catenin signaling suppresses bleomycin-induced pulmonary fibrosis by attenuating the expression of TGF-β1 and FGF-2.
Chen X, Shi C, Meng X, Zhang K, Li X, Wang C, Xiang Z, Hu K, Han X. Chen X, et al. Exp Mol Pathol. 2016 Aug;101(1):22-30. doi: 10.1016/j.yexmp.2016.04.003. Epub 2016 Apr 23. Exp Mol Pathol. 2016. PMID: 27112840 Free PMC article. - Overexpression of cathepsin K during silica-induced lung fibrosis and control by TGF-beta.
van den Brûle S, Misson P, Bühling F, Lison D, Huaux F. van den Brûle S, et al. Respir Res. 2005 Jul 27;6(1):84. doi: 10.1186/1465-9921-6-84. Respir Res. 2005. PMID: 16045809 Free PMC article. - Cathepsins in oral diseases: mechanisms and therapeutic implications.
Jiang H, Dong Z, Xia X, Li X. Jiang H, et al. Front Immunol. 2023 Jun 2;14:1203071. doi: 10.3389/fimmu.2023.1203071. eCollection 2023. Front Immunol. 2023. PMID: 37334378 Free PMC article. Review.
Cited by
- Protective effects and mechanism of curcumin in animal models of pulmonary fibrosis: a preclinical systematic review and meta-analysis.
Hanyu F, Zheng H, Jiaqi W, Tairan D, Yiyuanzi Z, Qiwen Y, Ying L, Hongchun Z, Lu L. Hanyu F, et al. Front Pharmacol. 2023 Oct 13;14:1258885. doi: 10.3389/fphar.2023.1258885. eCollection 2023. Front Pharmacol. 2023. PMID: 37900163 Free PMC article. - Roles of Nrf2/HO-1 and ICAM-1 in the Protective Effect of Nano-Curcumin against Copper-Induced Lung Injury.
Sarawi WS, Alhusaini AM, Alghibiwi HK, Alsaab JS, Hasan IH. Sarawi WS, et al. Int J Mol Sci. 2023 Sep 12;24(18):13975. doi: 10.3390/ijms241813975. Int J Mol Sci. 2023. PMID: 37762280 Free PMC article. - Renoprotective Roles of Curcumin.
Yaribeygi H, Maleki M, Majeed M, Jamialahmadi T, Sahebkar A. Yaribeygi H, et al. Adv Exp Med Biol. 2021;1328:531-544. doi: 10.1007/978-3-030-73234-9_38. Adv Exp Med Biol. 2021. PMID: 34981504 - Curcumin inhibition of bleomycin-induced changes in lung collagen synthesis, deposition and assembly.
Durairaj P, Venkatesan S, Narayanan V, Babu M. Durairaj P, et al. Mol Biol Rep. 2021 Dec;48(12):7775-7785. doi: 10.1007/s11033-021-06790-3. Epub 2021 Oct 13. Mol Biol Rep. 2021. PMID: 34643929 - Phenolic compounds disrupt spike-mediated receptor-binding and entry of SARS-CoV-2 pseudo-virions.
Goc A, Sumera W, Rath M, Niedzwiecki A. Goc A, et al. PLoS One. 2021 Jun 17;16(6):e0253489. doi: 10.1371/journal.pone.0253489. eCollection 2021. PLoS One. 2021. PMID: 34138966 Free PMC article.
References
- Smith MR, Gangireddy SR, Narala VR, Hogaboam CM, Standiford TJ, Christensen PJ, Kondapi AK, Reddy RC. Curcumin inhibits fibrosis-related effects in IPF fibroblasts and in mice following bleomycin-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2010;298(5):L616–625. doi: 10.1152/ajplung.00002.2009. - DOI - PMC - PubMed
- Zhao CJ, Niu JZ, Wang JF, Zhou G, Tang BH. [Effects of curcumin on bleomycin-induced damages in pulmonary functions in rats] Zhongguo Zhong Yao Za Zhi. 2008;33(12):1434–1438. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical