Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer - PubMed (original) (raw)
Clinical Trial
. 2012 Feb 9;366(6):520-9.
doi: 10.1056/NEJMoa1109653. Epub 2011 Dec 7.
Mario Campone, Martine Piccart, Howard A Burris 3rd, Hope S Rugo, Tarek Sahmoud, Shinzaburo Noguchi, Michael Gnant, Kathleen I Pritchard, Fabienne Lebrun, J Thaddeus Beck, Yoshinori Ito, Denise Yardley, Ines Deleu, Alejandra Perez, Thomas Bachelot, Luc Vittori, Zhiying Xu, Pabak Mukhopadhyay, David Lebwohl, Gabriel N Hortobagyi
Affiliations
- PMID: 22149876
- PMCID: PMC5705195
- DOI: 10.1056/NEJMoa1109653
Clinical Trial
Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer
José Baselga et al. N Engl J Med. 2012.
Abstract
Background: Resistance to endocrine therapy in breast cancer is associated with activation of the mammalian target of rapamycin (mTOR) intracellular signaling pathway. In early studies, the mTOR inhibitor everolimus added to endocrine therapy showed antitumor activity.
Methods: In this phase 3, randomized trial, we compared everolimus and exemestane versus exemestane and placebo (randomly assigned in a 2:1 ratio) in 724 patients with hormone-receptor-positive advanced breast cancer who had recurrence or progression while receiving previous therapy with a nonsteroidal aromatase inhibitor in the adjuvant setting or to treat advanced disease (or both). The primary end point was progression-free survival. Secondary end points included survival, response rate, and safety. A preplanned interim analysis was performed by an independent data and safety monitoring committee after 359 progression-free survival events were observed.
Results: Baseline characteristics were well balanced between the two study groups. The median age was 62 years, 56% had visceral involvement, and 84% had hormone-sensitive disease. Previous therapy included letrozole or anastrozole (100%), tamoxifen (48%), fulvestrant (16%), and chemotherapy (68%). The most common grade 3 or 4 adverse events were stomatitis (8% in the everolimus-plus-exemestane group vs. 1% in the placebo-plus-exemestane group), anemia (6% vs. <1%), dyspnea (4% vs. 1%), hyperglycemia (4% vs. <1%), fatigue (4% vs. 1%), and pneumonitis (3% vs. 0%). At the interim analysis, median progression-free survival was 6.9 months with everolimus plus exemestane and 2.8 months with placebo plus exemestane, according to assessments by local investigators (hazard ratio for progression or death, 0.43; 95% confidence interval [CI], 0.35 to 0.54; P<0.001). Median progression-free survival was 10.6 months and 4.1 months, respectively, according to central assessment (hazard ratio, 0.36; 95% CI, 0.27 to 0.47; P<0.001).
Conclusions: Everolimus combined with an aromatase inhibitor improved progression-free survival in patients with hormone-receptor-positive advanced breast cancer previously treated with nonsteroidal aromatase inhibitors. (Funded by Novartis; BOLERO-2 ClinicalTrials.gov number, NCT00863655.).
Figures
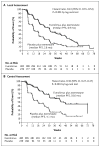
Figure 1. Kaplan–Meier Plot of Progression-free Survival
Panel A shows progression-free survival on the basis of local assessment of radiographic studies, and Panel B shows central assessment. PFS denotes progression-free survival.
Figure 2. Consistency of Results for Progression-free Survival across the Various Subgroups
Scores for Eastern Cooperative Oncology Group (ECOG) performance status range from 0 to 5, with 0 indicating that the patient is fully active, 1 indicating that the patient is restricted in physically strenuous activity but is ambulatory and able to carry out work of a light or sedentary nature, and 2 indicating that the patient is ambulatory and capable of all self-care but unable to work. The number of patients may not add up to 724 owing to missing baseline data. The size of each square is proportional to the number of patients in the subgroup. The data are shown on a semi-logarithmic scale.
Comment in
- Everolimus in HR-positive advanced breast cancer.
Massarweh S, Croley J, Weiss H. Massarweh S, et al. N Engl J Med. 2012 May 3;366(18):1738-9; author reply 1739-40. doi: 10.1056/NEJMc1202719. N Engl J Med. 2012. PMID: 22551139 No abstract available. - Everolimus in HR-positive advanced breast cancer.
Tartarone A, Lerose R, Aieta M. Tartarone A, et al. N Engl J Med. 2012 May 3;366(18):1739; author reply 1739-40. doi: 10.1056/NEJMc1202719. N Engl J Med. 2012. PMID: 22551140 No abstract available.
Similar articles
- Everolimus plus exemestane in postmenopausal patients with HR(+) breast cancer: BOLERO-2 final progression-free survival analysis.
Yardley DA, Noguchi S, Pritchard KI, Burris HA 3rd, Baselga J, Gnant M, Hortobagyi GN, Campone M, Pistilli B, Piccart M, Melichar B, Petrakova K, Arena FP, Erdkamp F, Harb WA, Feng W, Cahana A, Taran T, Lebwohl D, Rugo HS. Yardley DA, et al. Adv Ther. 2013 Oct;30(10):870-84. doi: 10.1007/s12325-013-0060-1. Epub 2013 Oct 25. Adv Ther. 2013. PMID: 24158787 Free PMC article. Clinical Trial. - Combination of everolimus with trastuzumab plus paclitaxel as first-line treatment for patients with HER2-positive advanced breast cancer (BOLERO-1): a phase 3, randomised, double-blind, multicentre trial.
Hurvitz SA, Andre F, Jiang Z, Shao Z, Mano MS, Neciosup SP, Tseng LM, Zhang Q, Shen K, Liu D, Dreosti LM, Burris HA, Toi M, Buyse ME, Cabaribere D, Lindsay MA, Rao S, Pacaud LB, Taran T, Slamon D. Hurvitz SA, et al. Lancet Oncol. 2015 Jul;16(7):816-29. doi: 10.1016/S1470-2045(15)00051-0. Epub 2015 Jun 16. Lancet Oncol. 2015. PMID: 26092818 Clinical Trial. - The BOLERO-2 trial: the addition of everolimus to exemestane in the treatment of postmenopausal hormone receptor-positive advanced breast cancer.
Beaver JA, Park BH. Beaver JA, et al. Future Oncol. 2012 Jun;8(6):651-7. doi: 10.2217/fon.12.49. Future Oncol. 2012. PMID: 22764762 Free PMC article. Clinical Trial.
Cited by
- Breast cancer in 2012: New drugs, new knowledge, new targets.
Chavez-MacGregor M, Gonzalez-Angulo AM. Chavez-MacGregor M, et al. Nat Rev Clin Oncol. 2013 Feb;10(2):75-6. doi: 10.1038/nrclinonc.2012.236. Epub 2013 Jan 8. Nat Rev Clin Oncol. 2013. PMID: 23296111 Review. No abstract available. - Targeting the human epidermal growth factor receptor 2 pathway in breast cancer.
Damodaran S, Olson EM. Damodaran S, et al. Hosp Pract (1995). 2012 Oct;40(4):7-15. doi: 10.3810/hp.2012.10.997. Hosp Pract (1995). 2012. PMID: 23299030 Free PMC article. - Challenges in the management of advanced, ER-positive, HER2-negative breast cancer.
Hart CD, Migliaccio I, Malorni L, Guarducci C, Biganzoli L, Di Leo A. Hart CD, et al. Nat Rev Clin Oncol. 2015 Sep;12(9):541-52. doi: 10.1038/nrclinonc.2015.99. Epub 2015 May 26. Nat Rev Clin Oncol. 2015. PMID: 26011489 Review. - BOLERO-2 - will this change practice in advanced breast cancer?
Johnston SR. Johnston SR. Breast Cancer Res. 2012 Jun 19;14(3):311. doi: 10.1186/bcr3126. Breast Cancer Res. 2012. PMID: 22713135 Free PMC article. - Estrogen receptor mutations and their role in breast cancer progression.
Alluri PG, Speers C, Chinnaiyan AM. Alluri PG, et al. Breast Cancer Res. 2014 Dec 12;16(6):494. doi: 10.1186/s13058-014-0494-7. Breast Cancer Res. 2014. PMID: 25928204 Free PMC article. Review.
References
- Smith IE, Dowsett M. Aromatase inhibitors in breast cancer. N Engl J Med. 2003;348:2431–42. - PubMed
- Mouridsen H, Gershanovich M, Sun Y, et al. Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: results of a phase III study of the International Letrozole Breast Cancer Group. J Clin Oncol. 2001;19:2596–606. [Erratum, J Clin Oncol 2001;19:3302.] - PubMed
- Nabholtz JM, Buzdar A, Pollak M, et al. Anastrozole is superior to tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: results of a North American multicenter randomized trial. J Clin Oncol. 2000;18:3758–67. - PubMed
- Bonneterre J, Thürlimann B, Robertson JF, et al. Anastrozole versus tamoxifen as first-line therapy for advanced breast cancer in 668 postmenopausal women: results of the Tamoxifen or Arimidex Randomized Group Efficacy and Tolerability study. J Clin Oncol. 2000;18:3748–57. - PubMed
- Mauri D, Pavlidis N, Polyzos NP, Ioannidis JP. Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: meta-analysis. J Natl Cancer Inst. 2006;98:1285–91. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous