Probiotic bacteria induce maturation of intestinal claudin 3 expression and barrier function - PubMed (original) (raw)
Probiotic bacteria induce maturation of intestinal claudin 3 expression and barrier function
Ravi M Patel et al. Am J Pathol. 2012 Feb.
Erratum in
- Corrections.
[No authors listed] [No authors listed] Am J Pathol. 2012 Mar;180(3):1324. doi: 10.1016/j.ajpath.2011.12.010. Epub 2012 Feb 16. Am J Pathol. 2012. PMID: 32455526 Free PMC article. No abstract available.
Abstract
An immature intestinal epithelial barrier may predispose infants and children to many intestinal inflammatory diseases, such as infectious enteritis, inflammatory bowel disease, and necrotizing enterocolitis. Understanding the factors that regulate gut barrier maturation may yield insight into strategies to prevent these intestinal diseases. The claudin family of tight junction proteins plays an important role in regulating epithelial paracellular permeability. Previous reports demonstrate that rodent intestinal barrier function matures during the first 3 weeks of life. We show that murine paracellular permeability markedly decreases during postnatal maturation, with the most significant change occurring between 2 and 3 weeks. Here we report for the first time that commensal bacterial colonization induces intestinal barrier function maturation by promoting claudin 3 expression. Neonatal mice raised on antibiotics or lacking the toll-like receptor adaptor protein MyD88 exhibit impaired barrier function and decreased claudin 3 expression. Furthermore, enteral administration of either live or heat-killed preparations of the probiotic Lactobacillus rhamnosus GG accelerates intestinal barrier maturation and induces claudin 3 expression. However, live Lactobacillus rhamnosus GG increases mortality. Taken together, these results support a vital role for intestinal flora in the maturation of intestinal barrier function. Probiotics may prevent intestinal inflammatory diseases by regulating intestinal tight junction protein expression and barrier function. The use of heat-killed probiotics may provide therapeutic benefit while minimizing adverse effects.
Copyright © 2012 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures
Figure 1
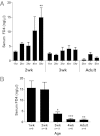
Murine intestinal epithelial barrier tightens by 3 weeks of postnatal age. A: Time course of intestinal permeability as determined by serum FD4 concentration measured 1 to 5 hours after oral gavage in mice at the ages of 2 and 3 weeks and in adult mice. Data are the mean ± SEM from at least three experimental repeats per condition. **P < 0.01 versus the 1-hour time point. B: Ontogeny of intestinal permeability, as measured by serum FD4 concentration measured 5 hours after gavage administration during the first 8 weeks of life. Data are the mean ± SEM from at least three experimental repeats per condition. *P < 0.05, **P < 0.01, and ***P < 0.001 versus the 2-week time point.
Figure 2
Claudin 3 gene expression peaks at 3 weeks in the murine intestine. Quantitative real-time RT-PCR of TJ protein genes undergoing developmental changes in expression in murine intestines collected during the first 8 weeks of life. A: Developmental survey of TJ protein gene expression. Bars represent fold induction when comparing mRNA levels in 3-week-old versus 2-day-old mice. B and C: mRNA expression of claudin 3 (B) and claudin 7 (C) over time. Data are expressed as fold change (mean ± SEM from at least three independent experiments) versus 2 days old (set to 1).
Figure 3
Mature claudin 3 protein expression and localization occurs at 3 weeks in the murine gut. A and B: Developmental expression of claudin 3 (CLDN3; A), CLDN7 (B), and occludin (OCLN) protein by using Western blot analysis in murine intestines collected during the first 8 weeks of life. Ad, adult. C and D: Band densitometry analysis of CLDN 3 (C) and CLDN 7 (D) protein expression, with protein density referenced to OCLN and normalized to 2 days postnatal age. *P < 0.05, ***P < 0.001 versus the 2-day time point. Data are the mean ± SEM of at least three independent experiments. E: Immunofluorescent localization of claudin 3 in 2-day-old, 2-week-old, 3-week-old, and Ad mice. Top panels: claudin 3 staining alone (green). Bottom panels: Nuclear counterstain (blue). Immature localization is noted primarily along the crypts in 2-day-old and 2-week-old mice (arrows). Data are representative of at least three independent experiments.
Figure 4
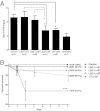
Maturation of intestinal claudin 3 expression and barrier function is MyD88 dependent. A: Immunofluorescent localization of claudin 3 in wild-type (control), MyD88−/−, or antibiotic-raised (Abx) 3-week-old mice. Top panels: claudin 3 staining alone (green). Bottom panels: Nuclear counterstain (blue). B: Expression of CLDN3 and occludin (OCLN) protein by immunoblot in murine intestines at the age of 3 weeks. C: Relative intestinal mRNA expression of claudin 3 in wild-type (control), MyD88−/−, or antibiotic-raised 3-week-old mice, as assayed by quantitative real-time RT-PCR. Data are the mean ± SEM from at least three experimental repeats per condition. D: Intestinal permeability, as measured by serum FD4 concentration 5 hours after oral gavage in wild-type (control), MyD88−/−, or antibiotic-raised 3-week-old mice. Data are the mean ± SEM from at least three experimental repeats per condition. *P < 0.05 versus control.
Figure 5
LGG accelerates barrier maturation. A: Intestinal permeability in 2-week-old mice, as measured by serum FD4 concentration (5 hours after oral gavage) in mice fed PBS alone (control) or with live (1 × 107 to 1 × 109 CFU) or heat-killed (1 × 109 CFU) LGG for 1 week. Intestinal permeability was compared with 3-week-old conventionally raised (CR) mice as a reference point for mature intestinal barrier function. Data are the mean ± SEM from at least three independent experiments. *P < 0.05, **P < 0.01. B: Kaplan-Meier survival curve for the same experimental groups as described in A. The percentage survival and the number of mice per group are indicated. ***P < 0.001 by log-rank Mantel-Cox test.
Figure 6
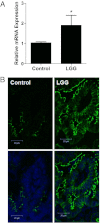
LGG induces claudin 3 expression. A: Relative intestinal mRNA expression of claudin 3, as assayed by quantitative real-time RT-PCR in mice gavage fed PBS alone (control) or with LGG for 1 week. Intestines were harvested at the age of 2 weeks. Data are the mean ± SEM from at least three experimental repeats per condition. *P < 0.05 versus control. B: Immunofluorescent localization of claudin 3 in mice treated with PBS alone (control) or with LGG (1 × 109 CFU) for 1 week. Top panels: claudin staining alone (green). Bottom panels: Nuclear counterstain (blue).
Similar articles
- Lactobacillus rhamnosus blocks inflammatory signaling in vivo via reactive oxygen species generation.
Lin PW, Myers LE, Ray L, Song SC, Nasr TR, Berardinelli AJ, Kundu K, Murthy N, Hansen JM, Neish AS. Lin PW, et al. Free Radic Biol Med. 2009 Oct 15;47(8):1205-11. doi: 10.1016/j.freeradbiomed.2009.07.033. Epub 2009 Aug 3. Free Radic Biol Med. 2009. PMID: 19660542 Free PMC article. - Lactobacillus GG restoration of the gliadin induced epithelial barrier disruption: the role of cellular polyamines.
Orlando A, Linsalata M, Notarnicola M, Tutino V, Russo F. Orlando A, et al. BMC Microbiol. 2014 Jan 31;14:19. doi: 10.1186/1471-2180-14-19. BMC Microbiol. 2014. PMID: 24483336 Free PMC article. - Lactobacillus rhamnosus CNCM I-3690 and the commensal bacterium Faecalibacterium prausnitzii A2-165 exhibit similar protective effects to induced barrier hyper-permeability in mice.
Laval L, Martin R, Natividad JN, Chain F, Miquel S, Desclée de Maredsous C, Capronnier S, Sokol H, Verdu EF, van Hylckama Vlieg JE, Bermúdez-Humarán LG, Smokvina T, Langella P. Laval L, et al. Gut Microbes. 2015;6(1):1-9. doi: 10.4161/19490976.2014.990784. Epub 2015 Jan 14. Gut Microbes. 2015. PMID: 25517879 Free PMC article. - Intestinal epithelial claudins: expression and regulation in homeostasis and inflammation.
Garcia-Hernandez V, Quiros M, Nusrat A. Garcia-Hernandez V, et al. Ann N Y Acad Sci. 2017 Jun;1397(1):66-79. doi: 10.1111/nyas.13360. Epub 2017 May 10. Ann N Y Acad Sci. 2017. PMID: 28493289 Free PMC article. Review. - Active and passive involvement of claudins in the pathophysiology of intestinal inflammatory diseases.
Barmeyer C, Fromm M, Schulzke JD. Barmeyer C, et al. Pflugers Arch. 2017 Jan;469(1):15-26. doi: 10.1007/s00424-016-1914-6. Epub 2016 Nov 30. Pflugers Arch. 2017. PMID: 27904960 Review.
Cited by
- Improvement of intestinal microbial structure in patients with cerebral infarction through in vitro fermentation of anthocyanins from Lycium ruthenicum Murray.
Qiu J, Ye B, Feng L. Qiu J, et al. Food Sci Nutr. 2024 Jul 28;12(10):7481-7491. doi: 10.1002/fsn3.4263. eCollection 2024 Oct. Food Sci Nutr. 2024. PMID: 39479706 Free PMC article. - Amelioration effect of Lactobacillus plantarum KFY02 on low-fiber diet-induced constipation in mice by regulating gut microbiota.
Yi R, Zhou X, Liu T, Xue R, Yang Z. Yi R, et al. Front Nutr. 2022 Aug 24;9:938869. doi: 10.3389/fnut.2022.938869. eCollection 2022. Front Nutr. 2022. PMID: 36091233 Free PMC article. - The role of intestinal epithelial barrier function in the development of NEC.
Halpern MD, Denning PW. Halpern MD, et al. Tissue Barriers. 2015 Jan 22;3(1-2):e1000707. doi: 10.1080/21688370.2014.1000707. eCollection 2015. Tissue Barriers. 2015. PMID: 25927016 Free PMC article. - Role of probiotics in short bowel syndrome in infants and children--a systematic review.
Reddy VS, Patole SK, Rao S. Reddy VS, et al. Nutrients. 2013 Mar 5;5(3):679-99. doi: 10.3390/nu5030679. Nutrients. 2013. PMID: 23462584 Free PMC article. Review. - Strengthening of the intestinal epithelial tight junction by Bifidobacterium bifidum.
Hsieh CY, Osaka T, Moriyama E, Date Y, Kikuchi J, Tsuneda S. Hsieh CY, et al. Physiol Rep. 2015 Mar;3(3):e12327. doi: 10.14814/phy2.12327. Physiol Rep. 2015. PMID: 25780093 Free PMC article.
References
- Sartor R.B. Microbial influences in inflammatory bowel diseases. Gastroenterology. 2008;134:577–594. - PubMed
- Clayburgh D.R., Shen L., Turner J.R. A porous defense: the leaky epithelial barrier in intestinal disease. Lab Invest. 2004;84:282–291. - PubMed
- Grave G.D., Nelson S.A., Walker W.A., Moss R.L., Dvorak B., Hamilton F.A., Higgins R., Raju T.N. New therapies and preventive approaches for necrotizing enterocolitis: report of a research planning workshop. Pediatr Res. 2007;62:510–514. - PubMed
- Alfaleh K., Bassler D. Probiotics for prevention of necrotizing enterocolitis in preterm infants. Cochrane Database Syst Rev. 2008;(1) CD005496. - PubMed
- Henry M.C., Moss R.L. Neonatal necrotizing enterocolitis. Semin Pediatr Surg. 2008;17:98–109. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HD059122/HD/NICHD NIH HHS/United States
- R01 DK 059888/DK/NIDDK NIH HHS/United States
- R24 DK064399/DK/NIDDK NIH HHS/United States
- T32DK007771/DK/NIDDK NIH HHS/United States
- HD059142-01S1/HD/NICHD NIH HHS/United States
- R01 HD059142/HD/NICHD NIH HHS/United States
- T32 DK007771/DK/NIDDK NIH HHS/United States
- R01 DK059888/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous