The matrisome: in silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices - PubMed (original) (raw)
The matrisome: in silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices
Alexandra Naba et al. Mol Cell Proteomics. 2012 Apr.
Abstract
The extracellular matrix (ECM) is a complex meshwork of cross-linked proteins providing both biophysical and biochemical cues that are important regulators of cell proliferation, survival, differentiation, and migration. We present here a proteomic strategy developed to characterize the in vivo ECM composition of normal tissues and tumors using enrichment of protein extracts for ECM components and subsequent analysis by mass spectrometry. In parallel, we have developed a bioinformatic approach to predict the in silico "matrisome" defined as the ensemble of ECM proteins and associated factors. We report the characterization of the extracellular matrices of murine lung and colon, each comprising more than 100 ECM proteins and each presenting a characteristic signature. Moreover, using human tumor xenografts in mice, we show that both tumor cells and stromal cells contribute to the production of the tumor matrix and that tumors of differing metastatic potential differ in both the tumor- and the stroma-derived ECM components. The strategy we describe and illustrate here can be broadly applied and, to facilitate application of these methods by others, we provide resources including laboratory protocols, inventories of ECM domains and proteins, and instructions for bioinformatically deriving the human and mouse matrisome.
Figures
Fig. 1.
Extracellular matrix enrichment protocol and proteomic analysis. A, ECM protein enrichment from total tissue sample. The extraction of intracellular components from [1] cytosolic, [2] nuclear, [3] membrane and [4] cytoskeletal fractions was monitored by immunoblotting for GAPDH (cytosol), histones (nucleus), the transferrin receptor (plasma membrane) and vimentin (cytoskeleton). The remaining insoluble fraction was enriched for ECM proteins (as shown in the fibronectin panel) and largely depleted for intracellular components. B, Proteomics workflow (see
Extended Experimental Procedures
).
Fig. 2.
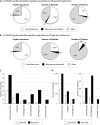
Characterization of the ECM of murine lung. A, Characterization of the ECM-enriched fraction from lung by LC-MS/MS proteomics. The pie charts display the results from one murine lung sample processed through the proteomics workflow. Proteins represented by at least two peptides were included in the analysis. Left panel shows the peptide abundance by precursor-ion MS signal corresponding to different protein categories. Middle panel: distribution in terms of numbers of peptides. Right panel: distribution in terms of numbers of proteins. The “core matrisome” division comprises ECM glycoproteins, collagens and proteoglycans; the “matrisome-associated” protein division encompasses ECM-affiliated proteins, ECM regulators and Secreted factors (see discussion in text and Fig. 3). MS data are presented in
supplemental Table S1
. B, Mass spectrometry results after peptide separation into 11 fractions by off-gel electrophoresis (OGE). The pie charts display the result of one murine lung sample processed through the proteomics workflow. Proteins represented by at least two peptides were included in the analysis. Left panel shows the peptide abundance by precursor-ion MS signal corresponding to the different divisions of the matrisome. Middle panel: distribution in terms of numbers of peptides. Right panel: distribution in terms of numbers of proteins. Note the increase in number of matrisome and matrisome-associated proteins detected in each category and the larger increase in nonECM proteins; that is, the increase in apparent “noise” relative to “signal” for ECM proteins. MS data are presented in
supplemental Table S4A
, sample 1. C, Comparison of the number of core matrisome and matrisome-associated proteins detected before and after peptide separation by OGE. D, Comparison of the number of peptides belonging to the core matrisome and matrisome-associated proteins before and after OGE.
Fig. 3.
Bioinformatics pipeline used to define the in silico core matrisome. A, List of the 55 InterPro domains used to define the core matrisome. This list was compiled based on prior knowledge, data from the literature and iterative query of UniProt as described in Extended Experimental Procedures (see also
supplemental Table S2A
). B, Schematic representation of the bioinformatic pipeline (see text,
Extended Experimental Procedures and supplemental Fig. S1
).
Fig. 4.
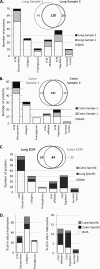
Reproducibility of the proteomic analysis. A, Two lung samples were analyzed through the proteomics pipeline and intersample variability was assessed. Venn diagram shows reproducibility in terms of number of matrisome proteins identified by two peptides in each of two independent samples. Bar chart represents the matrisome proteins detected in the two independent samples (see
supplemental Table S4A
). B, Two colon samples were analyzed through the proteomics pipeline and intersample variability was assessed. Venn diagram shows reproducibility in terms of number of matrisome proteins identified by two peptides in each of two independent samples. Bar chart represents the matrisome proteins detected in two independent samples (see
supplemental Table S4C
). C, Bar chart represents the overlap and tissue specificity for the lung and colon within each category of matrisome proteins. Venn diagram shows the number of matrisome proteins overlapping between the lung and colon and the number of tissue-specific matrisome proteins. Here, proteins are included only if they are found in both samples from a given tissue and represented by two peptides in at least one of them. D, Left panel: Comparison of the in vivo matrices of lung and colon core matrisome sets with the predicted in silico core matrisome. Right panel: Comparison of the in vivo lung and colon matrisome-associated sets of proteins with the predicted in silico matrisome-associated set of genes.
Fig. 5.
The tumor extracellular matrix is secreted by both tumor cells and stromal cells and differs with metastatic potential. A, Proteins secreted by A375 tumors and not by MA2 tumors. B, Proteins secreted by MA2 tumors and not by A375 tumors. C, Proteins expressed by both tumor types and by the same compartment in the two tumor types. D, Proteins expressed by both tumor types but by different compartments. Proteins are sorted by tumor type and by their origins: tumor (red), stroma (blue), or both (yellow: similar abundance of the human and mouse proteins, orange: human form is at least 5 times more abundant than the mouse form, green: the mouse form is at least 5 times more abundant than the human form in the two samples, ratios were calculated using the values indicated in column Y and values are given in column Z of
supplemental Table S6B and 6D
) and by category. UniProt accession numbers are given in
supplemental Table S6
(columns D and E). The number of shared peptides (common to human and mouse sequences) and of human- and mouse-specific peptides is given for each protein (data were extracted from columns V and W of
supplemental Table S6B and 6D
). BM indicates basement membrane proteins, H indicates proteins involved in hemostasis (i.e. plasma-derived - as expected, all of these are murine). Asterisks (*) indicate that, in addition to the identification of human- and mouse-specific peptides, the entries had a few peptides observed corresponding to distinct isoforms involving point mutations or exon deletions. For simplicity the isoforms are combined here. For detailed information, see
Extended Experimental Procedure and supplemental Table S6
.
Fig. 6.
Hyaluronan and proteoglycan link protein-1 (HAPLN1) is overexpressed in metastatic melanoma and secreted by the tumor cells. A, Numbers of nondistinguishing, species-specific and total peptides identified in the two metastatic tumor samples. In addition to peptides common to both the human and murine proteins, two human-specific peptides and no murine-specific peptide were detected, indicating that HAPLN1 is secreted by the tumor cells. Moreover, the two proteins are 96% identical in sequence so there are only a few diagnostic peptides. B, Sequence alignment of human and murine HAPLN1. Peptides identified by LC-MS/MS are highlighted in yellow if the peptide is identical in the two species and in pink if the peptide matches the human protein sequence. The identification of human-specific peptides and not closely related mouse peptides suggests that HAPLN1 is secreted exclusively by the tumor cells. C, Alignment of the sequence of the human protein fragment (amino acids 16 to 75) used to generate the anti-HAPLN1 antibody with the murine protein sequence (amino acids 16 to 77), which is 89% identical. D, HAPLN1 is up-regulated in MA2 tumors. Immunohistochemistry using anti-HAPLN1 antibody of A375 (upper panel) and MA2 (lower panel) tumor sections. Left panels show HAPLN1 staining, middle panel DAPI staining of the nuclei and right panels merge images.
Fig. 7.
The matrisome: in silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. We propose here to update and broaden the use of the term “matrisome”—first used by Martin and collaborators in 1984 to define supramolecular basement membrane complex (including collagen IV, laminin, nidogen, and proteoglycan) (57)—to define the universe of ECM proteins and their complexes that can contribute to ECM structures. The domain-based bioinformatic analyses defined a set of extracellular matrix proteins (core matrisome) and an additional set of matrisome-associated proteins within the human and mouse genomes. This in silico definition of the matrisome was further used to annotate the proteomic output. The combination of a robust definition of matrisomal proteins and a high throughput proteomics pipeline led to the identification of more than 100 extracellular matrix proteins within any given tissue or tumor.
Similar articles
- SWATH mass spectrometry as a tool for quantitative profiling of the matrisome.
Krasny L, Bland P, Kogata N, Wai P, Howard BA, Natrajan RC, Huang PH. Krasny L, et al. J Proteomics. 2018 Oct 30;189:11-22. doi: 10.1016/j.jprot.2018.02.026. Epub 2018 Mar 1. J Proteomics. 2018. PMID: 29501709 Free PMC article. - Comparison of extracellular matrix enrichment protocols for the improved characterization of the skin matrisome by mass spectrometry.
Dussoyer M, Page A, Delolme F, Rousselle P, Nyström A, Moali C. Dussoyer M, et al. J Proteomics. 2022 Jan 16;251:104397. doi: 10.1016/j.jprot.2021.104397. Epub 2021 Oct 20. J Proteomics. 2022. PMID: 34678517 - Deciphering the Kidney Matrisome: Identification and Quantification of Renal Extracellular Matrix Proteins in Healthy Mice.
Rende U, Ahn SB, Adhikari S, Moh ESX, Pollock CA, Saad S, Guller A. Rende U, et al. Int J Mol Sci. 2023 Feb 1;24(3):2827. doi: 10.3390/ijms24032827. Int J Mol Sci. 2023. PMID: 36769148 Free PMC article. - The extracellular matrix: Tools and insights for the "omics" era.
Naba A, Clauser KR, Ding H, Whittaker CA, Carr SA, Hynes RO. Naba A, et al. Matrix Biol. 2016 Jan;49:10-24. doi: 10.1016/j.matbio.2015.06.003. Epub 2015 Jul 8. Matrix Biol. 2016. PMID: 26163349 Free PMC article. Review. - Exploring the extracellular matrix in health and disease using proteomics.
Taha IN, Naba A. Taha IN, et al. Essays Biochem. 2019 Sep 13;63(3):417-432. doi: 10.1042/EBC20190001. Print 2019 Sep 13. Essays Biochem. 2019. PMID: 31462529 Review.
Cited by
- Mapping the molecular and structural specialization of the skin basement membrane for inter-tissue interactions.
Tsutsui K, Machida H, Nakagawa A, Ahn K, Morita R, Sekiguchi K, Miner JH, Fujiwara H. Tsutsui K, et al. Nat Commun. 2021 May 10;12(1):2577. doi: 10.1038/s41467-021-22881-y. Nat Commun. 2021. PMID: 33972551 Free PMC article. - The Burden of Post-Translational Modification (PTM)-Disrupting Mutations in the Tumor Matrisome.
Holstein E, Dittmann A, Kääriäinen A, Pesola V, Koivunen J, Pihlajaniemi T, Naba A, Izzi V. Holstein E, et al. Cancers (Basel). 2021 Mar 3;13(5):1081. doi: 10.3390/cancers13051081. Cancers (Basel). 2021. PMID: 33802493 Free PMC article. - The liver matrisome - looking beyond collagens.
Arteel GE, Naba A. Arteel GE, et al. JHEP Rep. 2020 Apr 18;2(4):100115. doi: 10.1016/j.jhepr.2020.100115. eCollection 2020 Aug. JHEP Rep. 2020. PMID: 32637906 Free PMC article. Review. - Cancer systems immunology.
Reticker-Flynn NE, Engleman EG. Reticker-Flynn NE, et al. Elife. 2020 Jul 13;9:e53839. doi: 10.7554/eLife.53839. Elife. 2020. PMID: 32657757 Free PMC article. Review. - Impaired Differentiation of Chronic Obstructive Pulmonary Disease Bronchial Epithelial Cells Grown on Bronchial Scaffolds.
Hedström U, Öberg L, Vaarala O, Dellgren G, Silverborn M, Bjermer L, Westergren-Thorsson G, Hallgren O, Zhou X. Hedström U, et al. Am J Respir Cell Mol Biol. 2021 Aug;65(2):201-213. doi: 10.1165/rcmb.2019-0395OC. Am J Respir Cell Mol Biol. 2021. PMID: 33882260 Free PMC article.
References
- Hynes R. O. (2002) Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687 - PubMed
- van der Flier A., Sonnenberg A. (2001) Function and interactions of integrins. Cell Tissue Res. 305, 285–298 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA014051/CA/NCI NIH HHS/United States
- U54 CA126515/CA/NCI NIH HHS/United States
- U54 CA163109/CA/NCI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases