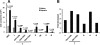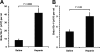Heparin-induced leukocytosis requires 6-O-sulfation and is caused by blockade of selectin- and CXCL12 protein-mediated leukocyte trafficking in mice - PubMed (original) (raw)
Heparin-induced leukocytosis requires 6-O-sulfation and is caused by blockade of selectin- and CXCL12 protein-mediated leukocyte trafficking in mice
Siyuan Zhang et al. J Biol Chem. 2012.
Abstract
Leukocytosis refers to an increase in leukocyte count above the normal range in the blood and is a common laboratory finding in patients. In many cases, the mechanisms underlying leukocytosis are not known. In this study, we examined the effects, the structural determinants, and the underlying mechanisms of heparin-induced leukocytosis, a side effect occurring in 0.44% of patients receiving heparin. We observed that heparin induced both lymphocytosis and neutrophilia, and the effects required heparin to be 6-O-sulfated but did not require its anticoagulant activity. Cell mobilization studies revealed that the lymphocytosis was attributable to a combination of blockage of lymphocyte homing and the release of thymocytes from the thymus, whereas the neutrophilia was caused primarily by neutrophil release from the bone marrow and demargination in the vasculature. Mechanistic studies revealed that heparin inhibits L- and P-selectin, as well as the chemokine CXCL12, leading to leukocytosis. Heparin is known to require 6-O-sulfate to inhibit L- and P-selectin function, and in this study we observed that 6-O-sulfate is required for its interaction with CXCL12. We conclude that heparin-induced leukocytosis requires glucosamine 6-O-sulfation and is caused by blockade of L-selectin-, P-selectin-, and CXCL12-mediated leukocyte trafficking.
Figures
FIGURE 1.
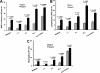
Heparin-induced leukocytosis is mainly attributable to lymphocytosis and neutrophilia. A and B, subpopulation distribution of leukocytes in cell numbers (A) and alteration in ratios over saline control (B). The leukocytosis is mainly attributable to a massive increase of circulating lymphocytes (lymphocytosis) and neutrophils (neutrophilia), which account for roughly 62 and 33% of the total leukocyte elevation in peripheral blood, respectively. The data were summarized from three sets of experiments with 8–12 mice per group. Each bar represents the average value ± S.E. The statistical analysis was carried out by paired Student's t test in comparison with saline treatment. WBC, white blood cells; L, lymphocytes; M, monocytes; N, neutrophils; T, T lymphocytes; B, B lymphocytes.
FIGURE 2.
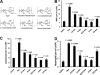
Leukocytosis induced by differently structured heparins (heparinoids). A, structure of heparinoids involved in this study. Representative disaccharide units of unfractionated heparin (Hep), _N_-desulfated and _N_-acetylated heparin (N-des), 2-O/3-_O_-desulfated heparin (2/3-des), _N_-/2-O/3-_O_-desulfated heparin (N/2/3-des), 6-_O_-desulfated heparin (6-des), and carboxyl-reduced heparin (CR-hep). B and D, heparinoid-induced elevation of leukocytes in blood was assessed for leukocytosis (B), lymphocytosis (C), and neutrophilia (D). The data were summarized from three sets of experiments with 8–12 mice per group. Each bar represents the average value ± S.E. The statistical analysis was carried out by paired Student's t test in comparison with saline treatment. WBC, white blood cells.
FIGURE 3.
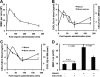
Heparin blocks lymphocyte homing to peripheral lymph nodes and releases thymocytes from thymus. A and B, lymphocytes homing to peripheral lymph nodes. CSFE+-labeled lymphocytes were injected into mice via tail vein, and their homing to lymph nodes was assessed 90 min post-heparin injection by quantifying CSFE+-lymphocytes in lymph nodes (A) and remaining in circulation (B). C, heparin released thymocytes from thymus. Thymocytes were intrathymically labeled with FITC 30 min prior to heparin injection, and FITC+ cells in blood were quantified 90 min post-heparin injection. The data were summarized from two sets of experiments with 4–5 mice per group. Each bar represents the average value ± S.E. The statistical analysis was carried out by paired Student's t test in comparison with saline treatment. PLN, peripheral lymph node; LN, lymph node.
FIGURE 4.
Heparin released neutrophils from the bone marrow and demarginated neutrophils in the vasculature. A, neutrophil release from BM into blood circulation. BrdU-labeled BM neutrophils in blood as Gr-1+/BrdU+ cell were quantified 90 min post-heparin injection. B, heparin mobilized neutrophils from marginating pool to circulating pool in the vasculature. Transfused BrdU-labeled neutrophils (BrdU+/Gr-1+) were allowed to reach the dynamic equilibrium of distribution between circulating and marginating pools, and then the BrdU+/Gr-1+ cells in circulating pool were quantified 90 min post-heparin injection. The data were summarized from two sets of experiments with 5–6 mice per group. Each bar represents the average value ± S.E. The statistical analysis was carried by paired Student's t test in comparison with saline treatment.
FIGURE 5.
Heparin-induced leukocytosis depends on blockade of P- and L-selectin function. Mice deficient in selectin (L−/−, P−/−, and PL−/−) or selectin ligand (FucT-IV/VII−/−) were injected i.v. with heparin or saline and then analyzed for leukocytosis (A), lymphocytosis (B), and neutrophilia (C). The data were summarized from three sets of experiments with 6–10 mice per group. Each bar represents the average value ± S.E. The statistical analysis was carried out by paired Student's t test in comparison with saline treatment. WBC, white blood cells.
FIGURE 6.
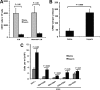
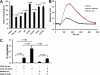
Heparin-induced leukocytosis depends on blockade of CXCL12 function. The kinetics of leukocytosis (A) and the levels of CXCL12α (B) and CXCL12β (C) in peripheral blood and in BM after heparin administration. The data were summarized from three experiments with 10 mice per group. D, blocking CXCL12 function with neutralizing antibody attenuated HIL response. The mice were preinjected i.v. with neutralizing anti-CXCL12 antibody prior to heparin or saline injection and then analyzed for HIL. The data were summarized from three experiments with 7–9 mice per group. Each bar represents the average value ± S.E. The statistical analysis was carried out by paired Student's t test. WBC, white blood cells. Ab, antibody.
FIGURE 7.
Heparin disrupted CXCL12 binding to heparan sulfate in a competitive and 6_-O-_sulfation-dependent manner and abolished the CXCL12 gradient-directed bone marrow cell migration. A, effects of heparinoids on the binding of CXCL12 to endothelial cell surface. CXCL12α was preincubated with heparan sulfate (HS), Hep, _N_-des-hep, 2/3-des-hep, 6-des-hep, or CR-hep. CXCL12α alone or the preincubated CXCL12a solution was incubated with untreated endothelial cells (control) or cells pretreated with heparinases. CXCL12α bound to the cell surface was detected by an ELISA. The data were summarized from three independent experiments. Bars represent mean ± S.E. The p values were calculated using the paired Student's t test in comparison with saline treatment. B, heparin but not 6-desulfated-heparin competitively inhibited CXCL12 binding to immobilized heparin in the SPR assay. CXCL12α was premixed with Hep or 6-des-hep and then the mixture was injected over heparin-immobilized CM5 chip surface. The SPR sensorgram shown is representative of three experiments. C, CXCL12 induced transendothelial cell migration of BM cells via both chemotaxis and chemokinesis, and heparin inhibited the CXCL12-induced chemotaxis of BM cells. The transendothelial cell migration assay was carried out with BM cells placed in the upper chamber and CXCL12 supplemented in the lower chamber (to determine chemotaxis) or immobilized to both abluminal and luminal surfaces (to determine chemokinesis). The BM cells that migrated into the lower chamber were counted. To determine the effect of heparin on CXCL12-induced chemotaxis of BM cells, heparin was added in both upper and lower chamber prior to initiation of cell migration. The data were summarized from three experiments. Each bar represents the average value ± S.E. The statistical analysis was carried by paired Student's t test.
Similar articles
- The "in and out" of glucosamine 6-O-sulfation: the 6th sense of heparan sulfate.
El Masri R, Seffouh A, Lortat-Jacob H, Vivès RR. El Masri R, et al. Glycoconj J. 2017 Jun;34(3):285-298. doi: 10.1007/s10719-016-9736-5. Epub 2016 Nov 3. Glycoconj J. 2017. PMID: 27812771 Review. - Heparin's anti-inflammatory effects require glucosamine 6-O-sulfation and are mediated by blockade of L- and P-selectins.
Wang L, Brown JR, Varki A, Esko JD. Wang L, et al. J Clin Invest. 2002 Jul;110(1):127-36. doi: 10.1172/JCI14996. J Clin Invest. 2002. PMID: 12093896 Free PMC article. - Inhibition of selectin-mediated cell adhesion and prevention of acute inflammation by nonanticoagulant sulfated saccharides. Studies with carboxyl-reduced and sulfated heparin and with trestatin a sulfate.
Xie X, Rivier AS, Zakrzewicz A, Bernimoulin M, Zeng XL, Wessel HP, Schapira M, Spertini O. Xie X, et al. J Biol Chem. 2000 Nov 3;275(44):34818-25. doi: 10.1074/jbc.M001257200. J Biol Chem. 2000. PMID: 10944519 - The influence of various structural parameters of semisynthetic sulfated polysaccharides on the P-selectin inhibitory capacity.
Fritzsche J, Alban S, Ludwig RJ, Rubant S, Boehncke WH, Schumacher G, Bendas G. Fritzsche J, et al. Biochem Pharmacol. 2006 Aug 14;72(4):474-85. doi: 10.1016/j.bcp.2006.05.006. Epub 2006 May 13. Biochem Pharmacol. 2006. PMID: 16780802 - PSGL-1 function in immunity and steady state homeostasis.
Carlow DA, Gossens K, Naus S, Veerman KM, Seo W, Ziltener HJ. Carlow DA, et al. Immunol Rev. 2009 Jul;230(1):75-96. doi: 10.1111/j.1600-065X.2009.00797.x. Immunol Rev. 2009. PMID: 19594630 Review.
Cited by
- Improving vasculoprotective effects of MSCs in coronary microvessels - benefits of 3D culture, sub-populations and heparin.
Bumroongthai K, Kavanagh DPJ, Genever P, Kalia N. Bumroongthai K, et al. Front Immunol. 2023 Oct 24;14:1257497. doi: 10.3389/fimmu.2023.1257497. eCollection 2023. Front Immunol. 2023. PMID: 37954606 Free PMC article. - Characterization of the interaction between Robo1 and heparin and other glycosaminoglycans.
Zhang F, Moniz HA, Walcott B, Moremen KW, Linhardt RJ, Wang L. Zhang F, et al. Biochimie. 2013 Dec;95(12):2345-53. doi: 10.1016/j.biochi.2013.08.018. Epub 2013 Aug 28. Biochimie. 2013. PMID: 23994753 Free PMC article. - Quantitative phosphoproteomics analysis reveals broad regulatory role of heparan sulfate on endothelial signaling.
Qiu H, Jiang JL, Liu M, Huang X, Ding SJ, Wang L. Qiu H, et al. Mol Cell Proteomics. 2013 Aug;12(8):2160-73. doi: 10.1074/mcp.M112.026609. Epub 2013 May 6. Mol Cell Proteomics. 2013. PMID: 23649490 Free PMC article. - In-vitro suppression of IL-6 and IL-8 release from human pulmonary epithelial cells by non-anticoagulant fraction of enoxaparin.
Shastri MD, Stewart N, Horne J, Peterson GM, Gueven N, Sohal SS, Patel RP. Shastri MD, et al. PLoS One. 2015 May 11;10(5):e0126763. doi: 10.1371/journal.pone.0126763. eCollection 2015. PLoS One. 2015. PMID: 25961885 Free PMC article. - The "in and out" of glucosamine 6-O-sulfation: the 6th sense of heparan sulfate.
El Masri R, Seffouh A, Lortat-Jacob H, Vivès RR. El Masri R, et al. Glycoconj J. 2017 Jun;34(3):285-298. doi: 10.1007/s10719-016-9736-5. Epub 2016 Nov 3. Glycoconj J. 2017. PMID: 27812771 Review.
References
- Opdenakker G., Fibbe W. E., Van Damme J. (1998) The molecular basis of leukocytosis. Immunol. Today 19, 182–189 - PubMed
- Abramson N., Melton B. (2000) Leukocytosis. Basics of clinical assessment. Am. Fam. Physician 62, 2053–2060 - PubMed
- Bradfield J. W., Born G. V. (1974) Lymphocytosis produced by heparin and other sulfated polysaccharides in mice and rats. Cell. Immunol. 14, 22–32 - PubMed
- Sasaki S. (1967) Production of lymphocytosis by polysaccharide polysulfates (heparinoids). Nature 214, 1041–1042 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01HL093339/HL/NHLBI NIH HHS/United States
- R01 HL093339/HL/NHLBI NIH HHS/United States
- P41 GM103390/GM/NIGMS NIH HHS/United States
- RR005351/RR/NCRR NIH HHS/United States
- R01AI37113/AI/NIAID NIH HHS/United States
- R01 AI037113/AI/NIAID NIH HHS/United States
- P41 RR005351/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases