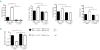Hepatic hypoxia-inducible factor-2 down-regulates hepcidin expression in mice through an erythropoietin-mediated increase in erythropoiesis - PubMed (original) (raw)
Hepatic hypoxia-inducible factor-2 down-regulates hepcidin expression in mice through an erythropoietin-mediated increase in erythropoiesis
Maria Mastrogiannaki et al. Haematologica. 2012 Jun.
Abstract
Background: Iron metabolism, regulated by the iron hormone hepcidin, and oxygen homeostasis, dependent on hypoxia-inducible factors, are strongly interconnected. We previously reported that in mice in which both liver hypoxia-inducible factors-1 and -2 are stabilized (the hepatocyte von Hippel-Lindau knockout mouse model), hepcidin expression was strongly repressed and we hypothesized that hypoxia-inducible factor-2 could be the major regulatory component contributing to the hepcidin down-regulation.
Design and methods: We generated and analyzed hepatocyte-specific knockout mice harboring either hypoxia-inducible factor-2α deficiency (Hif2a knockout) or constitutive hypoxia-inducible factor-2α stabilization (Vhlh/Hif1a knockout) and ex vivo systems (primary hepatocyte cultures). Hif2a knockout mice were fed an iron-deficient diet for 2 months and Vhlh/Hif1a knockout mice were treated with neutralizing erythropoietin antibody.
Results: We demonstrated that hypoxia-inducible factor-2 is dispensable in hepcidin gene regulation in the context of an adaptive response to iron-deficiency anemia. However, its overexpression in the double Vhlh/Hif1a hepatocyte-specific knockout mice indirectly down-regulates hepcidin expression through increased erythropoiesis and erythropoietin production. Experiments in primary hepatocytes confirmed the non-autonomous role of hypoxia-inducible factor-2 in hepcidin regulation.
Conclusions: While our results indicate that hypoxia-inducible factor-2 is not directly involved in hepcidin repression, they highlight the contribution of hepatic hypoxia-inducible factor-2 to the repression of hepcidin through erythropoietin-mediated increased erythropoiesis, a result of potential clinical interest.
Figures
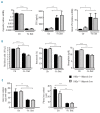
Figure 1.
Mice with HIF2-α-deficient liver respond normally to diet-induced iron deficiency. Hif2a _l_ox/loxAlbumin Cre-and _Hif2a_lox/loxAlbumin Cre+ littermates were fed an iron deficient (-Fe diet) or control diet (Ctr) for 2 months after weaning. (A) Hepatic hepcidin and renal erythropoietin (EPO) relative mRNA expression normalized to cyclophilin-A mRNA. Results are expressed as a fold change compared to the _Hif2a_lox/loxAlbumin Cre-mice on a control diet. EPO in plasma of WT and _Hif2a_lox/loxAlbumin Cre+ mice fed an iron deficient or control diet. (B) Hematologic parameters. (C) Liver and plasma iron contents. n ≥ 4 for each group.
Figure 2.
Characterization of _Vhlh_lox/lox_Hif1a_lox/loxAlbumin Cre+ mice. (A) Body, spleen and liver weight of _Vhlh_lox/lox Hif1a_lox/_loxAlbumin Cre+ (KO) and control mice (Ctr). n = 4. Plasma iron in _Vhlh_lox/lox _Hif1a_lox/loxAlbumin Cre+ (KO) and control mice (Ctr). n=5. (B) Relative mRNA expression normalized to cyclophilin-A mRNA in the liver of _Vhlh_lox/lox _Hif1a_lox/loxAlbumin Cre+ compared to Ctr. Ctr: n=9; KO : n=8. (C) Hepatic and renal erythropoietin mRNA (Ctr : n=11; KO : n=6) and plasma erythropoietin levels in _Vhlh_lox/lox_Hif1a_lox/loxAlbumin Cre+ (KO) compared with levels in control lit-termates (Ctr). Ctr: n=31; KO: n=14. (D) Hemoglobin and reticulocyte counts in _Vhlh_lox/lox _Hif1a_lox/loxAlbumin Cre+ (KO) and control mice (Ctr). n ≥ 3 for each group.
Figure 3.
Inhibition of erythropoietic activity in _Vhlh_lox/lox_Hif1a_lox/loxAlbumin _Cre_+ mice normalizes liver hepcidin expression. Mice were injected every day with 300 μL NaCl or rabbit anti-erythropoietin serum (a-EPO) for 5 days and sacrificed 16 h after the last injection. (A) Reticulocytes, red blood cells, hemoglobin and hematocrit counts. (B) Relative hepcidin mRNA expression in the liver expressed as a fold difference compared with that in NaCl injected control (Ctr) mice. Ctr: NaCl (n=10), Ctr: Anti-EPO (n=9), KO: NaCl (n=3), KO: Anti-EPO (n=5)
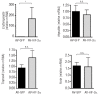
Figure 4.
HIF-2α overexpression does not repress hepcidin expression in primary hepatocytes. Infection of wild-type primary hepatocytes with a control GFP-adenovirus (AV-GFP) (black bar) or with a HIF2-α-overexpressing adenovirus (AV-HIF-2α) (gray bar) at a multiplicity of infection (MOI) of 10. Relative mRNA expression normalized to cyclophilin-A. Representation of three independent experiments performed in triplicate.
Similar articles
- Hypoxia-inducible factor regulates hepcidin via erythropoietin-induced erythropoiesis.
Liu Q, Davidoff O, Niss K, Haase VH. Liu Q, et al. J Clin Invest. 2012 Dec;122(12):4635-44. doi: 10.1172/JCI63924. Epub 2012 Nov 1. J Clin Invest. 2012. PMID: 23114598 Free PMC article. - The hypoxia-inducible factor-C/EBPα axis controls ethanol-mediated hepcidin repression.
Anderson ER, Taylor M, Xue X, Martin A, Moons DS, Omary MB, Shah YM. Anderson ER, et al. Mol Cell Biol. 2012 Oct;32(19):4068-77. doi: 10.1128/MCB.00723-12. Epub 2012 Aug 6. Mol Cell Biol. 2012. PMID: 22869521 Free PMC article. - Regulation of type II transmembrane serine proteinase TMPRSS6 by hypoxia-inducible factors: new link between hypoxia signaling and iron homeostasis.
Lakhal S, Schödel J, Townsend AR, Pugh CW, Ratcliffe PJ, Mole DR. Lakhal S, et al. J Biol Chem. 2011 Feb 11;286(6):4090-7. doi: 10.1074/jbc.M110.173096. Epub 2010 Oct 21. J Biol Chem. 2011. PMID: 20966077 Free PMC article. - Hypoxic regulation of erythropoiesis and iron metabolism.
Haase VH. Haase VH. Am J Physiol Renal Physiol. 2010 Jul;299(1):F1-13. doi: 10.1152/ajprenal.00174.2010. Epub 2010 May 5. Am J Physiol Renal Physiol. 2010. PMID: 20444740 Free PMC article. Review. - The mutual crosstalk between iron and erythropoiesis.
Camaschella C, Pagani A, Silvestri L, Nai A. Camaschella C, et al. Int J Hematol. 2022 Aug;116(2):182-191. doi: 10.1007/s12185-022-03384-y. Epub 2022 May 27. Int J Hematol. 2022. PMID: 35618957 Review.
Cited by
- Iron homeostasis and ferroptosis in human diseases: mechanisms and therapeutic prospects.
Ru Q, Li Y, Chen L, Wu Y, Min J, Wang F. Ru Q, et al. Signal Transduct Target Ther. 2024 Oct 14;9(1):271. doi: 10.1038/s41392-024-01969-z. Signal Transduct Target Ther. 2024. PMID: 39396974 Free PMC article. Review. - Iron Status and Physical Performance in Athletes.
Solberg A, Reikvam H. Solberg A, et al. Life (Basel). 2023 Oct 2;13(10):2007. doi: 10.3390/life13102007. Life (Basel). 2023. PMID: 37895389 Free PMC article. Review. - Mechanisms controlling cellular and systemic iron homeostasis.
Galy B, Conrad M, Muckenthaler M. Galy B, et al. Nat Rev Mol Cell Biol. 2024 Feb;25(2):133-155. doi: 10.1038/s41580-023-00648-1. Epub 2023 Oct 2. Nat Rev Mol Cell Biol. 2024. PMID: 37783783 Review. - Myeloid Hif2α is not essential to maintain systemic iron homeostasis.
Jain C, Parimi S, Huang W, Hannifin S, Singhal R, Das NK, Lee KE, Shah YM. Jain C, et al. Exp Hematol. 2023 Sep-Oct;125-126:25-36.e1. doi: 10.1016/j.exphem.2023.08.001. Epub 2023 Aug 8. Exp Hematol. 2023. PMID: 37562670 Free PMC article.
References
- Weidemann A, Johnson RS. Nonrenal regulation of EPO synthesis. Kidney Int. 2009;75(7):682–8. - PubMed
- Semenza GL. Involvement of oxygen-sensing pathways in physiologic and pathologic erythropoiesis. Blood. 2009;114(10):2015–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources