Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis - PubMed (original) (raw)
Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis
Bin Zhao et al. Genes Dev. 2012.
Abstract
Cell attachment to the extracellular matrix (ECM) is crucial to cell physiology such as polarity, motility, and proliferation. In normal cells, loss of attachment to the ECM induces a specific type of apoptosis, termed anoikis. Resistance to anoikis in cancer cells promotes their survival in circulation and dispersion to distant anatomic sites, leading to tumor metastasis. The Yes-associated protein (YAP) transcription coactivator is a human oncogene and a key regulator of organ size. The Hippo tumor suppressor pathway phosphorylates and inhibits YAP. However, little is known about the signals that regulate the Hippo pathway. Here we report that through cytoskeleton reorganization, cell detachment activates the Hippo pathway kinases Lats1/2 and leads to YAP phosphorylation and inhibition. The detachment-induced YAP inactivation is required for anoikis in nontransformed cells, whereas in cancer cells with deregulation of the Hippo pathway, knockdown of YAP and TAZ restores anoikis. Furthermore, we provided evidence that Lats1/2 expression level is indeed significantly down-regulated in metastatic prostate cancer. Our findings provide a novel connection between cell attachment and anoikis through the Hippo pathway and have important implications in cancer therapeutics.
Figures
Figure 1.
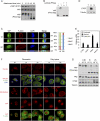
YAP phosphorylation, subcellular localization, and activity are regulated by cell attachment status. (A) Cell attachment induces dephosphorylation of YAP. MCF10A cells were trypsinized and attached onto fibronectin-coated petri dishes for the indicated time. Cells were then lysed, and cell lysates were analyzed by Western blots with the indicated specific antibodies. Phos-tag-containing SDS-PAGE gels were used as indicated. (B) Cell detachment strongly induces YAP phosphorylation. MCF10A cells were trypsinized and attached onto fibronectin-coated petri dishes for 2 h. Attached cells were either directly lysed (A) or trypsinized again before being collected and lysed (T). Aliquots of samples were further treated with λ protein phosphatase as indicated. Cell lysates were resolved on Phos-tag-containing SDS-PAGE gels, and anti-YAP antibody was used for Western blot analysis. (C) Cell attachment induces nuclear translocation of YAP. MCF10A cells were trypsinized and attached onto fibronectin-coated coverglasses for the indicated time (in minutes). Cells were then fixed and stained with anti-YAP antibody. Cell boundaries were visualized by staining of F-actin with rhodamine-phalloidin. Both XY views and XZ views from confocal imaging are presented in the left panel. Cells in five random views (∼100 cells) were quantified for YAP localization and are shown in the right panel. (D) Cell attachment activates CTGF expression. High cell density HeLa cells were trypsinized and lysed (T) or further cultured onto fibronectin-coated petri dishes (A) or onto ultralow attachment plates in suspension (S) for 4 h. Cell lysates were examined by Western blot analysis using the indicated antibodies. (E) Cell attachment activates YAP target gene expression. Experiments were similar to that in D. RNA was extracted, and expression of YAP target genes was analyzed by quantitative RT–PCR. (F) Attachment to fibronectin- or polylysine-coated supporting materials similarly induces nuclear translocation of YAP. MCF10A cells were trypsinized and attached onto fibronectin- or polylysine-coated coverglasses for the indicated time in serum-free medium. Cells were then fixed and stained with the indicated antibodies for vinculin, microtubule, or YAP, and with Alexa Fluor 488-conjugated phalloidin for F-actin. Cell nuclei were stained with DAPI. (G) Attachment to fibronectin- or polylysine-coated supporting materials similarly activates YAP and TAZ. Experiments were similar to that in F. Cells lysates were analyzed by Western blots. Phos-tag-containing SDS-PAGE gels were used as indicated. The asterisk indicates a nonspecific band.
Figure 2.
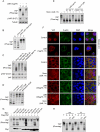
YAP is regulated by the actin and microtubule cytoskeletons. (A) Cell attachment-induced YAP dephosphorylation requires actin polymerization. MCF10A cells were trypsinized (T) and attached onto fibronectin-coated petri dishes (A) for 80 min. Latrunculin B was added as indicated at the time of plating. Cell lysates were analyzed by Western blots as indicated. (B) Microtubule organization is required for cell detachment-induced YAP phosphorylation. MCF10A cells were trypsinized and attached onto fibronectin-coated petri dishes for 2 h. Cells were either directly lysed (A) or trypsinized again before being collected and lysed (T). Nocodazole (Noco) and taxol were added to cell culture medium 15 min before and during trypsinization as indicated. (C) Disruption of the microtubule decreases YAP phosphorylation. MCF10A cells cultured to 80% confluence were treated with the indicated chemicals in culture medium. Cells were then lysed and analyzed by Western blots. (D) Disruption of the actin cytoskeleton induces YAP phosphorylation. MCF10A cells were trypsinized and attached onto fibronectin-coated petri dishes for 2 h, followed by addition of the indicated reagents, and were further cultured for another hour before being harvested for Western blot analysis. (E) F-actin is required for YAP dephosphorylation induced by microtubule depolymerization. MCF10A cells were cultured to confluence and treated with inhibitors in cultured medium as indicated. Cell lysates were resolved on Phos-tag-containing SDS-PAGE gels, and anti-YAP antibody was used for Western blotting. Data were cropped from the same exposure of the same blot. (F) The actin and microtubule cytoskeletons regulate YAP subcellular localization. MCF10A cells were cultured onto fibronectin-coated coverglasses at low or high cell density. Cells were treated with the indicated inhibitors before fixation. Samples were then stained with anti-YAP antibody for endogenous YAP, Alexa Fluor 488-conjugated phalloidin for F-actin, and DAPI for cell nuclei. (G) Rho family small GTPases induce YAP dephosphorylation. HEK293 cells were cotransfected with the indicated plasmids. Cells were then lysed and analyzed by Western blots. (H) Rho mediates cell attachment-induced YAP dephosphorylation. MCF10A cells were trypsinized and then attached to fibronectin- or polylysine-coated petri dishes for 2 h in the presence of Rho inhibitor C3 (2 μg/mL) or Rac inhibitor NSC23766 (100 μM). Cell lysates were resolved on Phos-tag-containing SDS-PAGE gels, and anti-YAP antibody was used for Western blotting.
Figure 3.
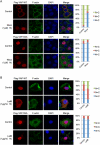
The cytoskeleton regulates YAP subcellular localization through the Hippo pathway phosphorylation sites. (A) Subcellular localization of YAP wild type but not the 5SA mutant is regulated by microtubule cytoskeleton integrity. HeLa cells were transfected with Flag-YAP wild type or 5SA mutant and then cultured onto fibronectin-coated coverglasses at high cell density. Cells were treated with 25 μg/mL LMB 3 h before fixation. In the last hour, nocodazole was added as indicated. Cells were stained with anti-Flag tag antibody for YAP, Alexa Fluor 488-conjugated phalloidin for F-actin, and DAPI for cell nuclei. Cells in five random views (∼100 cells) were quantified for YAP localization and are shown in the right panels. (B) Localization of YAP wild type but not the 5SA mutant is responsive to actin cytoskeleton integrity. HeLa cells were transfected as in A and then cultured onto fibronectin-coated coverglasses at low cell density. Cells were treated with latrunculin B 1 h before fixation as indicated. Cells were stained with anti-Flag tag antibody for YAP, Alexa Fluor 488-conjugated phalloidin for F-actin, and DAPI for cell nuclei. Cells in five random views (∼100 cells) were quantified for YAP localization and are shown in the right panels.
Figure 4.
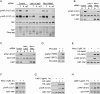
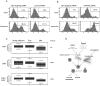
Cell attachment and cytoskeleton remodeling regulate Lats1/2 kinase activity. (A) The Hippo pathway kinases are crucial in cell detachment-induced YAP phosphorylation. HeLa cells were transfected with the indicated siRNAs twice with a 24-h interval. Cells either were directly lysed or went through the following treatment: trypsinized (T), trypsinized and then attached to fibronectin-coated petri dishes for 2 h (A), or further trypsinized after attachment (reT). Samples were analyzed by Western blots as indicated. S and L indicate short and long exposures, respectively. (B) A YAP kinase is activated by cell detachment. HeLa cells were transfected with the indicated siRNAs twice with a 24-h interval. Cells were replated onto fibronectin-coated petri dishes for 3 h and then directly lysed (A) or lysed following trypsinization (T). Cell lysates were then incubated with GST-YAP immobilized on glutathione resin. The resins were then washed and subjected to in vitro kinase assays. The products were analyzed by Western blots as indicated. (C) Lats1 is activated by cell detachment. Cell lysates were prepared as in B. Lats1 immunoprecipitated from the cell lysates was subjected to in vitro kinase assays using GST-YAP purified from Escherichia coli as a substrate. The products were analyzed by Western blotting as indicated. (D) Cell detachment increases Lats2 phosphorylation. HeLa cells transfected with HA-Lats2 were replated onto fibronectin-coated petri dishes for 3 h and then directly lysed (A) or lysed following trypsinization (T). HA-Lats2 was immunoprecipitated with anti-HA antibody and then subjected to Western blot analysis as indicated. (E) Lats2 is inhibited by microtubule disruption. HeLa cells transfected with HA-Lats2 and cultured at high cell density were treated as indicated. Untransfected cells were used as negative control. HA-Lats2 immunoprecipitated with anti-HA antibody was subjected to in vitro kinase assay using GST-YAP as a substrate. The products were analyzed by Western blotting as indicated. (F) Endogenous Lats1 is inhibited by disruption of the microtubule. Experiments were similar to that in E except that endogenous Lats1 was immunoprecipitated from untransfected HeLa cells. (G) Lats2 is activated by actin depolymerization. Experiments were similar to that in E except that HeLa cells were newly plated on fibronectin-coated petri dishes and treated with latrunculin B as indicated. (H) Endogenous Lats1 is activated by actin depolymerization. Experiments were similar to those in G except that endogenous Lats1 was immunoprecipitated from untransfected HeLa cells.
Figure 5.
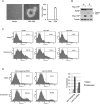
YAP inhibits detachment-induced anoikis. (A) Active YAP induces anchorage-independent growth in MCF10A cells. MCF10A cells infected with control vector or YAP-5SA were cultured in soft agar for 16 d before pictures were taken. Colony number was counted and is shown in the right panel. (B) YAP inhibits suspension-induced PARP cleavage. Control or YAP-expressing HMLE cells were cultured on tissue culture plates (A) or ultralow attachment plates in suspension (S) for 72 h. Cells were then harvested and analyzed by Western blotting with specific antibodies. (C) YAP inhibits anoikis. HMLE cells with control vector or ectopic expression of YAP wild type or 5SA mutant were cultured on tissue culture plates (Attach) or ultralow attachment plates in suspension for 72 h. Cells were then collected and stained with PE Annexin V and analyzed by FACS. Experiments were done in duplicate. (D) Knockdown of Lats1/2 inhibits anoikis in HMLE cells. HMLE cells were transfected with control siRNA or siRNAs targeting Lats1 and Lats2. Cells were then cultured on tissue culture plates (Attach) or ultralow attachment plates in suspension. After 72 h, cells were collected and stained with PE Annexin V and analyzed by FACS. Apoptosis rates were quantified from two independent experiments and are shown in the right panel.
Figure 6.
Inactivation of the Hippo pathway contributes to anoikis evasion and correlates with cancer metastasis. (A) Lats1/2 knockdown impairs anoikis in RWPE cells. RWPE cells were transfected with control siRNA or siRNAs targeting Lats1 and Lats2. Cells were then cultured on tissue culture plates (Attach) or ultralow attachment plates in suspension. After 72 h, cells were collected and stained with PE Annexin V and analyzed by FACS. Experiments were done in duplicate. (B) YAP/TAZ knockdown restores anoikis in ACHN cells. Experiments were similar to that in A except that siRNAs against YAP and TAZ as well as ACHN cells were used. Experiments were done in duplicate. (C) Lats1/2 down-regulation correlates with prostate cancer metastasis. Box plots showing the expression value of Lats1 and Lats2 in benign adjacent, localized (PCa), and metastatic (Met) prostate tumors. _P_-value measures the statistical difference between metastatic prostate cancer when compared with benign tissue and localized prostate cancer. (D) A model of the Hippo pathway mediating the cell attachment signal to anoikis and cancer metastasis.
Similar articles
- Regulation of the Hippo-YAP pathway by protease-activated receptors (PARs).
Mo JS, Yu FX, Gong R, Brown JH, Guan KL. Mo JS, et al. Genes Dev. 2012 Oct 1;26(19):2138-43. doi: 10.1101/gad.197582.112. Epub 2012 Sep 12. Genes Dev. 2012. PMID: 22972936 Free PMC article. - Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein.
Zhao B, Li L, Lu Q, Wang LH, Liu CY, Lei Q, Guan KL. Zhao B, et al. Genes Dev. 2011 Jan 1;25(1):51-63. doi: 10.1101/gad.2000111. Genes Dev. 2011. PMID: 21205866 Free PMC article. - Reciprocal regulation of YAP/TAZ by the Hippo pathway and the Small GTPase pathway.
Jang JW, Kim MK, Bae SC. Jang JW, et al. Small GTPases. 2020 Jul;11(4):280-288. doi: 10.1080/21541248.2018.1435986. Epub 2018 Apr 20. Small GTPases. 2020. PMID: 29457552 Free PMC article. Review. - A YAP/TAZ-induced feedback mechanism regulates Hippo pathway homeostasis.
Moroishi T, Park HW, Qin B, Chen Q, Meng Z, Plouffe SW, Taniguchi K, Yu FX, Karin M, Pan D, Guan KL. Moroishi T, et al. Genes Dev. 2015 Jun 15;29(12):1271-84. doi: 10.1101/gad.262816.115. Genes Dev. 2015. PMID: 26109050 Free PMC article. - Hippo-YAP/TAZ signaling in angiogenesis.
Park JA, Kwon YG. Park JA, et al. BMB Rep. 2018 Mar;51(3):157-162. doi: 10.5483/bmbrep.2018.51.3.016. BMB Rep. 2018. PMID: 29366443 Free PMC article. Review.
Cited by
- The role of the hippo pathway in melanocytes and melanoma.
Kim JE, Finlay GJ, Baguley BC. Kim JE, et al. Front Oncol. 2013 May 16;3:123. doi: 10.3389/fonc.2013.00123. eCollection 2013. Front Oncol. 2013. PMID: 23720711 Free PMC article. - Single-cell states versus single-cell atlases - two classes of heterogeneity that differ in meaning and method.
Janes KA. Janes KA. Curr Opin Biotechnol. 2016 Jun;39:120-125. doi: 10.1016/j.copbio.2016.03.015. Epub 2016 Apr 1. Curr Opin Biotechnol. 2016. PMID: 27042975 Free PMC article. Review. - The hippo pathway in heart development, regeneration, and diseases.
Zhou Q, Li L, Zhao B, Guan KL. Zhou Q, et al. Circ Res. 2015 Apr 10;116(8):1431-47. doi: 10.1161/CIRCRESAHA.116.303311. Circ Res. 2015. PMID: 25858067 Free PMC article. Review. - KRAS drives immune evasion in a genetic model of pancreatic cancer.
Ischenko I, D'Amico S, Rao M, Li J, Hayman MJ, Powers S, Petrenko O, Reich NC. Ischenko I, et al. Nat Commun. 2021 Mar 5;12(1):1482. doi: 10.1038/s41467-021-21736-w. Nat Commun. 2021. PMID: 33674596 Free PMC article. - Polarity-dependent distribution of angiomotin localizes Hippo signaling in preimplantation embryos.
Hirate Y, Hirahara S, Inoue K, Suzuki A, Alarcon VB, Akimoto K, Hirai T, Hara T, Adachi M, Chida K, Ohno S, Marikawa Y, Nakao K, Shimono A, Sasaki H. Hirate Y, et al. Curr Biol. 2013 Jul 8;23(13):1181-94. doi: 10.1016/j.cub.2013.05.014. Epub 2013 Jun 20. Curr Biol. 2013. PMID: 23791731 Free PMC article.
References
- Chan EH, Nousiainen M, Chalamalasetty RB, Schafer A, Nigg EA, Sillje HH 2005. The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene 24: 2076–2086 - PubMed
- Chiarugi P, Giannoni E 2008. Anoikis: A necessary death program for anchorage-dependent cells. Biochem Pharmacol 76: 1352–1364 - PubMed
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, et al. 2011. Role of YAP/TAZ in mechanotransduction. Nature 474: 179–183 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources