Mutations in FKBP14 cause a variant of Ehlers-Danlos syndrome with progressive kyphoscoliosis, myopathy, and hearing loss - PubMed (original) (raw)
. 2012 Feb 10;90(2):201-16.
doi: 10.1016/j.ajhg.2011.12.004. Epub 2012 Jan 19.
Cecilia Giunta, Birgit Krabichler, Franz Rüschendorf, Nicoletta Zoppi, Marina Colombi, Reginald E Bittner, Susana Quijano-Roy, Francesco Muntoni, Sebahattin Cirak, Gudrun Schreiber, Yaqun Zou, Ying Hu, Norma Beatriz Romero, Robert Yves Carlier, Albert Amberger, Andrea Deutschmann, Volker Straub, Marianne Rohrbach, Beat Steinmann, Kevin Rostásy, Daniela Karall, Carsten G Bönnemann, Johannes Zschocke, Christine Fauth
Affiliations
- PMID: 22265013
- PMCID: PMC3276673
- DOI: 10.1016/j.ajhg.2011.12.004
Mutations in FKBP14 cause a variant of Ehlers-Danlos syndrome with progressive kyphoscoliosis, myopathy, and hearing loss
Matthias Baumann et al. Am J Hum Genet. 2012.
Abstract
We report on an autosomal-recessive variant of Ehlers-Danlos syndrome (EDS) characterized by severe muscle hypotonia at birth, progressive scoliosis, joint hypermobility, hyperelastic skin, myopathy, sensorineural hearing impairment, and normal pyridinoline excretion in urine. Clinically, the disorder shares many features with the kyphoscoliotic type of EDS (EDS VIA) and Ullrich congenital muscular dystrophy. Linkage analysis in a large Tyrolean kindred identified a homozygous frameshift mutation in FKBP14 in two affected individuals. Based on the cardinal clinical characteristics of the disorder, four additional individuals originating from different European countries were identified who carried either homozygous or compound heterozygous mutations in FKBP14. FKBP14 belongs to the family of FK506-binding peptidyl-prolyl cis-trans isomerases (PPIases). ER-resident FKBPs have been suggested to act as folding catalysts by accelerating cis-trans isomerization of peptidyl-prolyl bonds and to act occasionally also as chaperones. We demonstrate that FKBP14 is localized in the endoplasmic reticulum (ER) and that deficiency of FKBP14 leads to enlarged ER cisterns in dermal fibroblasts in vivo. Furthermore, indirect immunofluorescence of FKBP14-deficient fibroblasts indicated an altered assembly of the extracellular matrix in vitro. These findings suggest that a disturbance of protein folding in the ER affecting one or more components of the extracellular matrix might cause the generalized connective tissue involvement in this disorder. FKBP14 mutation analysis should be considered in all individuals with apparent kyphoscoliotic type of EDS and normal urinary pyridinoline excretion, in particular in conjunction with sensorineural hearing impairment.
Copyright © 2012 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures
Figure 1
Pedigrees of the Families The circles indicate females, the squares males, and the triangles spontaneous abortions. Filled symbols indicate affected individuals with proven homozygous or compound heterozygous FKBP14 mutations. The index person P1 in family I (individual V-2) is indicated by an arrow. The deceased, probably affected, member of family I is indicated as P∗ (individual IV-1).
Figure 2
Clinical Phenotype Clinical findings in individuals with FKBP14-deficient EDS. (A and B) Severe kyphoscoliosis in P1 (age 15 years) (A) and lumbar scoliosis in P6 (3 years) (B). (C–G) Genu recurvatum in P3 (C) and distally pronounced joint hypermobility in P3 (D, 10 years), P5 (E, 3 years), and P6 (F and G, 3 years). (H and I) Muscle hypotonia and weakness in P6 (H, 3 months) and P5 (I, 1.5 years). (J) Moderate atrophy of intrinsic hand muscles in P2 (47 years). (K and L) Hyperelastic skin of the forearm of P3 (K, 10 years) and the neck region of P1 (L, 16 years). (M) Follicular hyperkeratosis in the pretibial region of P3 (10 years).
Figure 3
Muscle MRI, Histomorphology, and Ultrastructure of Muscle and Skin (A and B) Histomorphology of a muscle biopsy taken from the quadriceps muscle of P6 at the age of 1 year showing mild changes with increased variation in muscle fiber diameter: Gomori trichrome (A) and NADH-TR stain (B). (C and D) Histochemistry (NADH-TR) of a muscle biopsy from the paraspinal muscle of P3 at the age of 6 years shows areas with central activity defects of oxidative enzymes (C), corresponding to focal rearrangements of myofibrils in electron microscopy (D). (E and F) Transmission electron microscopy of muscle (longitudinal sections) in P1 and P4. (E) Electron microscopy in P1 shows focal rearrangements of myofibrils with irregular Z lines (arrow) (quadriceps muscle, at the age of 2 years). (F) Electron microscopy in P4 shows bifurcation of sarcomeres, small zones of Z-band streaming (arrow), and some disorganized myofibrils (dorsal muscle, at the age of 12 years). (G) Transverse T1-weighted muscle MRI cross sections of the right thigh and lower leg in P4 at the age of 12 years show abnormal signals in the rectus femoris (RF), vastus lateralis (VL), and soleus (So) muscles indicating fatty degeneration of muscle tissue. (H and I) Two images of transmission electron microscopy of a skin biopsy of P1 show abnormally enlarged ER cisterns filled with a flocculent material; collagen fibrils are normal in shape and diameter. Scale bars represent 20 μm in light microscopy and 2 μm in electron microscopy.
Figure 4
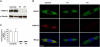
Subcellular Localization of FKBP14, Western Blot Analysis, and Nonsense-Mediated Decay (A) Immunoblot shows absence of FKBP14 in skin fibroblasts of P1 and P3 in comparison to controls. (B) Confocal immunofluorescence microscopy shows colocalization of FKBP14 (red) and HSP47 (green) in control fibroblasts. In fibroblasts from P1 and P3, FKBP14 is not detectable. Nuclei are counterstained by DAPI. Scale bar represents 10 μm. (C) qRT-PCR analysis of FKBP14 in P1, P3, and two control individuals. Relative expression was determined by the ΔΔCt method with HPRT1 as reference gene. Expression of FKBP14 was strongly reduced in patient fibroblasts, indicating nonsense-mediated decay.
Figure 5
Immunofluorescence of Extracellular Matrix Proteins in Cultured Skin Fibroblasts from Individuals with FKBP14 Deficiency Disarray of collagen types I, III, VI, fibronectin, tenascins, thrombospondin, and the integrin receptors α2β1 and α5β1 is demonstrated in FKBP14-deficient fibroblasts of P1 and P3 in comparison to control fibroblasts (one of two control cultures is shown; the scale bars represent 10 μm). In control cells, collagen type I was synthesized and mainly detected in the cytoplasm and only a few fibrils were assembled in the extracellular compartment. In FKBP14-deficient cells, collagen type I was reduced or not detectable in the cytoplasm and absent in the ECM. Collagen types III, V, and VI, fibronectin, and tenascins were assembled by control fibroblasts in distinct extracellular networks whereas FKBP14-deficient cells organized an extracellular network only for collagen type V and did not assemble a network for collagen type III and tenascins. The extracellular networks for collagen type VI and fibronectin were almost completely disorganized in FKBP14-deficient cells. In control cells, thrombospondin was detected either in the extracellular space or in the cytoplasm in the perinuclear ER; in contrast, FKBP14-deficient cells did not organize the protein in an extracellular network and only a few intracellular deposits were detected. The main receptors for collagens and fibronectin, α2β1 and α5β1 integrins, were not detectable in FKBP14-deficient cells.
Similar articles
- Transcriptome Profiling of Primary Skin Fibroblasts Reveal Distinct Molecular Features Between _PLOD1_- and _FKBP14_-Kyphoscoliotic Ehlers-Danlos Syndrome.
Lim PJ, Lindert U, Opitz L, Hausser I, Rohrbach M, Giunta C. Lim PJ, et al. Genes (Basel). 2019 Jul 8;10(7):517. doi: 10.3390/genes10070517. Genes (Basel). 2019. PMID: 31288483 Free PMC article. - A cohort of 17 patients with kyphoscoliotic Ehlers-Danlos syndrome caused by biallelic mutations in FKBP14: expansion of the clinical and mutational spectrum and description of the natural history.
Giunta C, Baumann M, Fauth C, Lindert U, Abdalla EM, Brady AF, Collins J, Dastgir J, Donkervoort S, Ghali N, Johnson DS, Kariminejad A, Koch J, Kraenzlin M, Lahiri N, Lozic B, Manzur AY, Morton JEV, Pilch J, Pollitt RC, Schreiber G, Shannon NL, Sobey G, Vandersteen A, van Dijk FS, Witsch-Baumgartner M, Zschocke J, Pope FM, Bönnemann CG, Rohrbach M. Giunta C, et al. Genet Med. 2018 Jan;20(1):42-54. doi: 10.1038/gim.2017.70. Epub 2017 Jun 15. Genet Med. 2018. PMID: 28617417 Free PMC article. - Further delineation of FKBP14-related Ehlers-Danlos syndrome: A patient with early vascular complications and non-progressive kyphoscoliosis, and literature review.
Dordoni C, Ciaccio C, Venturini M, Calzavara-Pinton P, Ritelli M, Colombi M. Dordoni C, et al. Am J Med Genet A. 2016 Aug;170(8):2031-8. doi: 10.1002/ajmg.a.37728. Epub 2016 May 5. Am J Med Genet A. 2016. PMID: 27149304 Review. - FKBP14 kyphoscoliotic Ehlers-Danlos Syndrome in adolescent patient: the first Colombian report.
Ruiz-Botero F, Ramírez-Montaño D, Pachajoa H. Ruiz-Botero F, et al. Arch Argent Pediatr. 2019 Jun 1;117(3):e274-e278. doi: 10.5546/aap.2019.eng.e274. Arch Argent Pediatr. 2019. PMID: 31063316 English, Spanish. - Ehlers-Danlos syndrome related to FKBP14 mutations: detailed cutaneous phenotype.
Bursztejn AC, Baumann M, Lipsker D. Bursztejn AC, et al. Clin Exp Dermatol. 2017 Jan;42(1):64-67. doi: 10.1111/ced.12983. Epub 2016 Nov 30. Clin Exp Dermatol. 2017. PMID: 27905128 Review.
Cited by
- Whole-exome sequencing provides insights into monogenic disease prevalence in Northwest Russia.
Barbitoff YA, Skitchenko RK, Poleshchuk OI, Shikov AE, Serebryakova EA, Nasykhova YA, Polev DE, Shuvalova AR, Shcherbakova IV, Fedyakov MA, Glotov OS, Glotov AS, Predeus AV. Barbitoff YA, et al. Mol Genet Genomic Med. 2019 Nov;7(11):e964. doi: 10.1002/mgg3.964. Epub 2019 Sep 3. Mol Genet Genomic Med. 2019. PMID: 31482689 Free PMC article. - The first case report of Kyphoscoliotic Ehlers-Danlos syndrome of chinese origin with a novel PLOD1 gene mutation.
Ni X, Jin C, Jiang Y, Wang O, Li M, Xing X, Xia W. Ni X, et al. BMC Med Genet. 2020 Oct 31;21(1):214. doi: 10.1186/s12881-020-01154-3. BMC Med Genet. 2020. PMID: 33129265 Free PMC article. - Orchestration of secretory protein folding by ER chaperones.
Gidalevitz T, Stevens F, Argon Y. Gidalevitz T, et al. Biochim Biophys Acta. 2013 Nov;1833(11):2410-24. doi: 10.1016/j.bbamcr.2013.03.007. Epub 2013 Mar 15. Biochim Biophys Acta. 2013. PMID: 23507200 Free PMC article. Review. - Functional diversity and pharmacological profiles of the FKBPs and their complexes with small natural ligands.
Galat A. Galat A. Cell Mol Life Sci. 2013 Sep;70(18):3243-75. doi: 10.1007/s00018-012-1206-z. Epub 2012 Dec 8. Cell Mol Life Sci. 2013. PMID: 23224428 Free PMC article. Review. - Genetics and signaling mechanisms of orofacial clefts.
Reynolds K, Zhang S, Sun B, Garland MA, Ji Y, Zhou CJ. Reynolds K, et al. Birth Defects Res. 2020 Nov;112(19):1588-1634. doi: 10.1002/bdr2.1754. Epub 2020 Jul 15. Birth Defects Res. 2020. PMID: 32666711 Free PMC article. Review.
References
- Steinmann B., Royce P.M., Superti-Furga A. The Ehlers-Danlos syndrome. In: Royce P.M., Steinmann B., editors. Connective Tissue and Its Heritable Disorders. 2nd ed. Wiley-Liss; New York: 2002. pp. 431–523.
- Beighton P., De Paepe A., Steinmann B., Tsipouras P., Wenstrup R.J., Ehlers-Danlos National Foundation (USA) and Ehlers-Danlos Support Group (UK) Ehlers-Danlos syndromes: revised nosology, Villefranche, 1997. Am. J. Med. Genet. 1998;77:31–37. - PubMed
- Kivirikko K.I., Pihlajaniemi T. Collagen hydroxylases and the protein disulfide isomerase subunit of prolyl 4-hydroxylases. Adv. Enzymol. Relat. Areas Mol. Biol. 1998;72:325–398. - PubMed
- Giunta C., Randolph A., Al-Gazali L.I., Brunner H.G., Kraenzlin M.E., Steinmann B. Nevo syndrome is allelic to the kyphoscoliotic type of the Ehlers-Danlos syndrome (EDS VIA) Am. J. Med. Genet. A. 2005;133A:158–164. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous