Mitochondrial apoptosis and FAK signaling disruption by a novel histone deacetylase inhibitor, HTPB, in antitumor and antimetastatic mouse models - PubMed (original) (raw)
doi: 10.1371/journal.pone.0030240. Epub 2012 Jan 18.
Tzu-Tang Wei, Yen-An Tang, Sin-Ming Huang, Wei-Ling Wen, Mei-Yu Chen, Hung-Chi Cheng, Santosh B Salunke, Ching-Shih Chen, Pinpin Lin, Chien-Tien Chen, Yi-Ching Wang
Affiliations
- PMID: 22279574
- PMCID: PMC3261198
- DOI: 10.1371/journal.pone.0030240
Mitochondrial apoptosis and FAK signaling disruption by a novel histone deacetylase inhibitor, HTPB, in antitumor and antimetastatic mouse models
Jiunn-Min Shieh et al. PLoS One. 2012.
Abstract
Background: Compound targeting histone deacetylase (HDAC) represents a new era in molecular cancer therapeutics. However, effective HDAC inhibitors for the treatment of solid tumors remain to be developed.
Methodology/principal findings: Here, we propose a novel HDAC inhibitor, N-Hydroxy-4-(4-phenylbutyryl-amino) benzamide (HTPB), as a potential chemotherapeutic drug for solid tumors. The HDAC inhibition of HTPB was confirmed using HDAC activity assay. The antiproliferative and anti-migratory mechanisms of HTPB were investigated by cell proliferation, flow cytometry, DNA ladder, caspase activity, Rho activity, F-actin polymerization, and gelatin-zymography for matrix metalloproteinases (MMPs). Mice with tumor xenograft and experimental metastasis model were used to evaluate effects on tumor growth and metastasis. Our results indicated that HTPB was a pan-HDAC inhibitor in suppressing cell viability specifically of lung cancer cells but not of the normal lung cells. Upon HTPB treatment, cell cycle arrest was induced and subsequently led to mitochondria-mediated apoptosis. HTPB disrupted F-actin dynamics via downregulating RhoA activity. Moreover, HTPB inhibited activity of MMP2 and MMP9, reduced integrin-β1/focal adhesion complex formation and decreased pericellular poly-fibronectin assemblies. Finally, intraperitoneal injection or oral administration of HTPB efficiently inhibited A549 xenograft tumor growth in vivo without side effects. HTPB delayed lung metastasis of 4T1 mouse breast cancer cells. Acetylation of histone and non-histone proteins, induction of apoptotic-related proteins and de-phosphorylation of focal adhesion kinase were confirmed in treated mice.
Conclusions/significance: These results suggested that intrinsic apoptotic pathway may involve in anti-tumor growth effects of HTPB in lung cancer cells. HTPB significantly suppresses tumor metastasis partly through inhibition of integrin-β1/FAK/MMP/RhoA/F-actin pathways. We have provided convincing preclinical evidence that HTPB is a potent HDAC targeted inhibitor and is thus a promising candidate for lung cancer chemotherapy.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
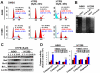
Figure 1. Effect of HTPB on cell viability and on the biomarkers associated with broad inhibition on numerous HDACs.
(A) Chemical structure of HTPB (upper left). Dose-dependent effects of HTPB on cell viability in IMR90, H1299, and A549 cells (lower left). Cells were treated with 0.5–10 µM of HTPB for 48 hours, and cell viability was assessed by MTT assay. A known HDAC inhibitor, SAHA, was used for comparison. (B) HTPB suppressed activities of class I (HDAC1 and HDAC8), class II (HDAC4 and HDAC6), and class IV (HDAC11) HDACs in A549 cells. Data represent mean ± SEM from three independent experiments. *** P<0.001. Dose-dependent effects (C) and time-dependent effects (D) of HTPB on the histone and non-histone proteins. SAHA was included for comparison. (E) HTPB induced acetylation of histone H3 and H4 without affecting the total protein levels of HDAC1 and HDAC 6. In addition, HTPB induced p21 protein expression in both A549 (p53 wild-type) and H1299 (p53 null) cells. The immunoblots shown are representatives of three independent experiments.
Figure 2. HTPB induces cell cycle arrest and apoptosis.
(A) The effects of HTPB on cell cycle distribution in A549 and H1299 cells. Cells were treated with 5 µM HTPB for indicated times and assessed by flow cytometry. The percentage of G2/M and sub-G1 fraction population is plotted in the histogram. G2/M arrest and sub-G1 induction are indicated by arrows. (B) HTPB caused apoptotic DNA ladders in A549 and H1299 cells treated with 5 µM HTPB for 48 hours. HTPB induced intrinsic apoptosis. Cells were treated with 5 µM HTPB for indicated times and cell lysates were subjected to Western blot analyses (C) and caspase activity assay (D). Pro-apoptotic proteins Bad and Bak were up-regulated and anti-apoptotic proteins Bcl-2 and Bcl-XL were down-regulated. Caspases-3 and -9 were up-regulated in both A549 and H1299 cells. Data represent mean ± SEM from three independent experiments. * P<0.05; ** P<0.01.
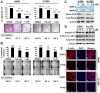
Figure 3. HTPB inhibits cancer cell migration via reduced activities of matrix metalloproteinases, RhoA, and focal adhesion complex.
(A) The image from trans-well migration assay and (B) wound-healing assay indicated that after 48 hours treatment at non-cytotoxic doses, HTPB inhibited migratory activity in a dose-dependent manner. * P<0.05; ** P<0.01; scale bars: 400 µm. (C) Gelatin-zymography assay and RhoA-GTP GST pull-down assay showed that MMP-2 and MMP-9 enzyme activities were suppressed and RhoA-GTP expression was reduced in A549 and H1299 cells after 2.5 µM HTPB treatment for 48 hours. (D) Expression of integrin-β1 and phosphorylation of FAK at Tyr-397 were down-regulated in H1299 and A549 cells after HTPB treatment for 48 hours at the indicated doses. (E) HTPB led to F-actin dysregulation by immunofluorescence analyses. Cells were treated with 5 µM HTPB for 48 hours, and then fixed and stained with phalloidin (F-actin). Scale bars: 40 µm.
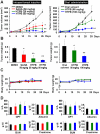
Figure 4. HTPB effectively inhibits A549 xenograft growth without significant side effects.
(A) Balb/c nude mice bearing the established A549 tumors (∼50 mm3) were treated with HTPB via intraperitoneal (left panel) or oral administration (right panel) for three weeks (3 days/week). A known HDAC inhibitor, SAHA, was used for comparison in intraperitoneal experiments. The tumor volumes of mice were measured twice weekly. Points, mean; bars, ±SEM. Three mice per group for intraperitoneal injection and five mice per group for oral treatment were used in the xenograft experiment. (B) The tumor weights of mice were measured. P values were for comparisons with DMSO or vehicle control (* P<0.05, ** P<0.01). (C) HTPB treatments did not cause significant body weight loss of tested animals. (D) Hematological biochemistry tests including GOT, GPT, albumin and creatinine were examined and the results showed no significant differences between HTPB treatment and DMSO or solvent control.
Figure 5. HTPB delays lung metastasis of 4T1-luc breast cancer cell in animal models.
(A) 4T1-luc mouse breast cancer cells were treated with 1.92 µM HTPB for 48 hours. HTPB did not significantly affect cell growth of 4T1-luc cells during the indicated treatments. (B) Fibronectin assembly on the surface of 4T1-luc cells measured by immunofluorescence analyses showed that HTPB treatment reduced pericellular poly-fibronectin assemblies. (C) The treated 4T1-luc cells were injected intravenously via tail vein into Balb/c mice and observed for the luciferase signals and photographed using IVIS50 for 13 days after drug treatment. HTPB significantly delayed lung metastasis.
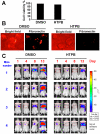
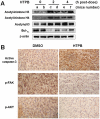
Figure 6. HTPB effectively induced protein acetylation, apoptosis and pFAK/pAKT inactivation in vivo.
(A) Mice bearing established (about 100∼200 mm3) A549 tumors were injected intraperitoneally with a single dose of HTPB at 50 mg/kg. After treatment for the indicated time, tumors from two representative mice of each time point (a–f) were harvested and subjected to Western blot using anti-actyl-histone H3, H4 and p53 and Bcl-XL antibodies. (B) Immunohistochemistry analyses were performed using antibody against cleaved-form of caspase-3, p-FAK and p-AKT (brownish color). Original magnification×200.
Similar articles
- A novel histone deacetylase inhibitor exhibits antitumor activity via apoptosis induction, F-actin disruption and gene acetylation in lung cancer.
Tang YA, Wen WL, Chang JW, Wei TT, Tan YH, Salunke S, Chen CT, Chen CS, Wang YC. Tang YA, et al. PLoS One. 2010 Sep 14;5(9):e12417. doi: 10.1371/journal.pone.0012417. PLoS One. 2010. PMID: 20856855 Free PMC article. - The novel indole compound SK228 induces apoptosis and FAK/Paxillin disruption in tumor cell lines and inhibits growth of tumor graft in the nude mouse.
Huang SM, Hsu PC, Chen MY, Li WS, More SV, Lu KT, Wang YC. Huang SM, et al. Int J Cancer. 2012 Aug 1;131(3):722-32. doi: 10.1002/ijc.26401. Epub 2011 Oct 20. Int J Cancer. 2012. PMID: 22015944 - Histone deacetylase inhibitors in cancer therapy.
Lane AA, Chabner BA. Lane AA, et al. J Clin Oncol. 2009 Nov 10;27(32):5459-68. doi: 10.1200/JCO.2009.22.1291. Epub 2009 Oct 13. J Clin Oncol. 2009. PMID: 19826124 Review. - Targeting Histone Modifications in Bone and Lung Metastatic Cancers.
Edwards CM, Johnson RW. Edwards CM, et al. Curr Osteoporos Rep. 2021 Jun;19(3):230-246. doi: 10.1007/s11914-021-00670-2. Epub 2021 Mar 15. Curr Osteoporos Rep. 2021. PMID: 33721181 Free PMC article. Review.
Cited by
- New Insights into the Implication of Epigenetic Alterations in the EMT of Triple Negative Breast Cancer.
Khaled N, Bidet Y. Khaled N, et al. Cancers (Basel). 2019 Apr 18;11(4):559. doi: 10.3390/cancers11040559. Cancers (Basel). 2019. PMID: 31003528 Free PMC article. Review. - The role and possible molecular mechanism of valproic acid in the growth of MCF-7 breast cancer cells.
Ma XJ, Wang YS, Gu WP, Zhao X. Ma XJ, et al. Croat Med J. 2017 Oct 31;58(5):349-357. doi: 10.3325/cmj.2017.58.349. Croat Med J. 2017. PMID: 29094813 Free PMC article. - Down-regulation of miR-675-5p contributes to tumor progression and development by targeting pro-tumorigenic GPR55 in non-small cell lung cancer.
He D, Wang J, Zhang C, Shan B, Deng X, Li B, Zhou Y, Chen W, Hong J, Gao Y, Chen Z, Duan C. He D, et al. Mol Cancer. 2015 Apr 1;14:73. doi: 10.1186/s12943-015-0342-0. Mol Cancer. 2015. PMID: 25889562 Free PMC article. - Epigenetics in non-small cell lung cancer: from basics to therapeutics.
Ansari J, Shackelford RE, El-Osta H. Ansari J, et al. Transl Lung Cancer Res. 2016 Apr;5(2):155-71. doi: 10.21037/tlcr.2016.02.02. Transl Lung Cancer Res. 2016. PMID: 27186511 Free PMC article. Review. - Regulation of autophagy and mitophagy by nutrient availability and acetylation.
Webster BR, Scott I, Traba J, Han K, Sack MN. Webster BR, et al. Biochim Biophys Acta. 2014 Apr 4;1841(4):525-34. doi: 10.1016/j.bbalip.2014.02.001. Epub 2014 Feb 11. Biochim Biophys Acta. 2014. PMID: 24525425 Free PMC article. Review.
References
- Lazebnik Y. What are the hallmarks of cancer? Nat Rev Cancer. 2010;10:232–233. - PubMed
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2010;100:57–70. - PubMed
- Siddiqa A, Marciniak R. Targeting the hallmarks of cancer. Cancer Biol Ther. 2008;7:740–741. - PubMed
- Levitzki A, Klein S. Signal transduction therapy of cancer. Mol Aspects Med. 2010;31:287–329. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous