A placebo-controlled trial of Korean red ginseng extract for preventing influenza-like illness in healthy adults - PubMed (original) (raw)
Randomized Controlled Trial
doi: 10.1186/1472-6882-12-10.
Min-Gul Kim, Mi-Ra Oh, Eun-Kyung Choi, Hyang-Im Back, Sun-Young Kim, Eun-Ok Park, Dae-Young Kwon, Hye-Jeong Yang, Min-Jeong Kim, Hee-Joo Kang, Ju-Hyung Lee, Kyung-Min Choi, Soo-Wan Chae, Chang-Seop Lee
Affiliations
- PMID: 22314101
- PMCID: PMC3297520
- DOI: 10.1186/1472-6882-12-10
Randomized Controlled Trial
A placebo-controlled trial of Korean red ginseng extract for preventing influenza-like illness in healthy adults
Ki-Chan Ha et al. BMC Complement Altern Med. 2012.
Abstract
Background: Standardized Korean red ginseng extract has become the best-selling influenza-like illness (ILI) remedy in Korea, yet much controversy regarding the efficacy of the Korean red ginseng (KRG) in reducing ILI incidence remains. The aim of the study is to assess the efficacy of the KRG extract on the ILI incidence in healthy adults.
Methods/design: We will conduct a randomized, double-blind, placebo-controlled study at the onset of the influenza seasons. A total of 100 subjects 30-70 years of age will be recruited from the general populations. The subjects will be instructed to take 9 capsules per day of either the KRG extract or a placebo for a period of 3 months. The primary outcome measure is to assess the frequency of ILI onset in participated subjects. Secondary variable measures will be included severity and duration of ILI symptoms. The ILI symptoms will be scored by subjects using a 4-point scale.
Discussion: This study is a randomized placebo controlled trial to evaluate the efficacy of the KRG extract compared to placebo and will be provided valuable new information about the clinical and physiological effects of the KRG extract on reduction of ILI incidence including flu and upper respiratory tract infections. The study has been pragmatically designed to ensure that the study findings can be implemented into clinical practice if KRG extract can be shown to be an effective reduction strategy in ILI incidence.
Trial registration: NCT01478009.
Figures
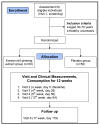
Figure 1
Flow diagram for study. Summary of the study flow.
Similar articles
- Preventive effect of Korean red ginseng for acute respiratory illness: a randomized and double-blind clinical trial.
Lee CS, Lee JH, Oh M, Choi KM, Jeong MR, Park JD, Kwon DY, Ha KC, Park EO, Lee N, Kim SY, Choi EK, Kim MG, Chae SW. Lee CS, et al. J Korean Med Sci. 2012 Dec;27(12):1472-8. doi: 10.3346/jkms.2012.27.12.1472. Epub 2012 Dec 7. J Korean Med Sci. 2012. PMID: 23255845 Free PMC article. Clinical Trial. - Efficacy of an extract of North American ginseng containing poly-furanosyl-pyranosyl-saccharides for preventing upper respiratory tract infections: a randomized controlled trial.
Predy GN, Goel V, Lovlin R, Donner A, Stitt L, Basu TK. Predy GN, et al. CMAJ. 2005 Oct 25;173(9):1043-8. doi: 10.1503/cmaj.1041470. CMAJ. 2005. PMID: 16247099 Free PMC article. Clinical Trial. - Effects of Korean red ginseng on sexual arousal in menopausal women: placebo-controlled, double-blind crossover clinical study.
Oh KJ, Chae MJ, Lee HS, Hong HD, Park K. Oh KJ, et al. J Sex Med. 2010 Apr;7(4 Pt 1):1469-77. doi: 10.1111/j.1743-6109.2009.01700.x. Epub 2010 Feb 5. J Sex Med. 2010. PMID: 20141583 Clinical Trial. - Ginseng for managing menopausal woman's health: A systematic review of double-blind, randomized, placebo-controlled trials.
Lee HW, Choi J, Lee Y, Kil KJ, Lee MS. Lee HW, et al. Medicine (Baltimore). 2016 Sep;95(38):e4914. doi: 10.1097/MD.0000000000004914. Medicine (Baltimore). 2016. PMID: 27661038 Free PMC article. Review. - Ginseng for Treating Hypertension: A Systematic Review and Meta-Analysis of Double Blind, Randomized, Placebo-Controlled Trials.
Lee HW, Lim HJ, Jun JH, Choi J, Lee MS. Lee HW, et al. Curr Vasc Pharmacol. 2017;15(6):549-556. doi: 10.2174/1570161115666170713092701. Curr Vasc Pharmacol. 2017. PMID: 28707603 Review.
Cited by
- A Review on Medicinal Plants Used in the Management of Respiratory Problems in Ethiopia over a Twenty-Year Period (2000-2021).
Haile AA, Tsegay BA, Seid A, Adnew W, Moges A. Haile AA, et al. Evid Based Complement Alternat Med. 2022 Jun 27;2022:2935015. doi: 10.1155/2022/2935015. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 35795271 Free PMC article. Review. - Evaluation of botanicals as potential COVID-19 symptoms terminator.
Caliskan UK, Karakus MM. Caliskan UK, et al. World J Gastroenterol. 2021 Oct 21;27(39):6551-6571. doi: 10.3748/wjg.v27.i39.6551. World J Gastroenterol. 2021. PMID: 34754152 Free PMC article. Review. - Pharmacological Efficacy of Ginseng against Respiratory Tract Infections.
Alsayari A, Muhsinah AB, Almaghaslah D, Annadurai S, Wahab S. Alsayari A, et al. Molecules. 2021 Jul 5;26(13):4095. doi: 10.3390/molecules26134095. Molecules. 2021. PMID: 34279434 Free PMC article. Review. - Complementary and alternative medicine use among outpatients during the 2015 MERS outbreak in South Korea: a cross-sectional study.
Hwang JH, Cho HJ, Im HB, Jung YS, Choi SJ, Han D. Hwang JH, et al. BMC Complement Med Ther. 2020 May 13;20(1):147. doi: 10.1186/s12906-020-02945-0. BMC Complement Med Ther. 2020. PMID: 32404092 Free PMC article. - Ginseng alleviates microbial infections of the respiratory tract: a review.
Iqbal H, Rhee DK. Iqbal H, et al. J Ginseng Res. 2020 Mar;44(2):194-204. doi: 10.1016/j.jgr.2019.12.001. Epub 2019 Dec 12. J Ginseng Res. 2020. PMID: 32148400 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical